Valve insufficiency. Causes
When people in the medical community talk about valve insufficiency, they mean a “breakage” of the valve, due to which it closes poorly or opens poorly (stenosis). The heart has 4 chambers and, accordingly, 4 valves that control the flow of blood from one chamber to another. When one valve fails, the others also become damaged over time.
Pulmonary valve insufficiency occurs when the leaflets do not close tightly.
Causes of deficiency:
- Congenital deficiency.
- Acquired pathology.
Acquired valve insufficiency develops in adults due to the following health problems:
- Infective endocarditis is inflammation of the inner lining of the heart.
- Carcinoid syndrome. In the syndrome, a small tumor in the intestine releases harmful substances that gradually destroy the right side of the heart and lungs. But this is a very rare disease.
- Rheumatism. With this inflammatory disease, the valves of the heart muscle are often damaged.
- Syphilis.
- Serious chest injury resulting in valve rupture.
- Drug use.
- Mitral stenosis.
- Presence of blood clots in the pulmonary trunk.
- Pickwick syndrome, its main symptom is problems with the lungs.
- Right ventricular dilatation due to tricuspid valve regurgitation.
Another important reason is long-term lung disease in smokers.
Here the reverse process is triggered - first pulmonary hypertension begins, and then, as a consequence, the functioning of the pulmonary valve is disrupted.
A pathology such as insufficiency can manifest itself in a mild degree and in a severe form, when surgical intervention is already necessary.
Types of deficiency
This pathology is divided into several subtypes. It depends on the origin of the disease and the nature of its course.
According to the time of occurrence (or origin) there are:
- congenital deficiency;
- acquired (usually due to infections, tumors, injuries).
Depending on the nature of the course, failure can be:
- acute (when a heart defect appears almost immediately after birth, since several valves and septa are affected);
- chronic (the disease manifests itself only after a few years).
There are other classifications. Failure can be organic (when it is caused by a pathology in the structure of the valve) or functional (when the flow of blood back into the heart is caused by an expansion of the artery trunk or the cavities of the heart).
Degrees of pulmonary valve regurgitation
The term “regurgitation” in medicine means that the heart valve does not close completely, causing blood to flow in the opposite direction. For example, when the pulmonary valve is damaged, blood moves from the artery to the right ventricle, causing it to become overfilled with blood. This defect at stage 1 does not significantly impair the functioning of the heart. In this case, hemodynamics are not disturbed, the thickness of the cardiac muscle of the right ventricle remains within normal limits.
But when grade 2 pulmonary valve regurgitation is detected, the person already has some health complaints. His right ventricle is already beginning to feel increased stress.
With pulmonary regurgitation of the 2nd degree, the heart becomes more and more damaged over time, so the system’s work no longer has the same synchronicity that it used to have, and the entire “mechanism” is gradually deteriorating.
Diagnostic methods
The diagnosis can be made taking into account the history, complaints, examination and examination of the patient. In the absence of clinical manifestations of insufficient blood supply, patients do not make complaints. Therefore, many people often do not even know that they have pulmonary regurgitation.
If there is a circulatory disorder, complaints may include rapid heartbeat, shortness of breath, sudden causeless changes in heart rate, pain in the left side of the chest, swelling of the extremities, especially in the evenings, pain in the peritoneum due to the growth of the liver.
Anamnesis makes it possible to identify chronic diseases, previous operations that could cause endocarditis, atrial injury, and find out whether the patient has taken narcotic injections.
Using instrumental diagnostic methods, accurate information about regurgitation can be obtained. Echocardiographic and electrocardiographic examination, as well as the Doppler method, are used. The cardiogram shows signs of congestion or enlargement of the right chambers of the heart, rhythm disturbances, and functionality of the pulmonary valve.
Using cardiac ultrasound, an assessment is made of the size of the organ, changes in its structure and size, the functioning of the left and right atria and ejection fraction. Doppler ultrasound helps determine whether there is pulmonary regurgitation at all, and what its degree is.
Laboratory tests show pathologies accompanied by regurgitation of the pulmonary artery and pulmonary valve: lipid metabolism disorders, positive tests for rheumatism, the presence of the Wassermann reaction.
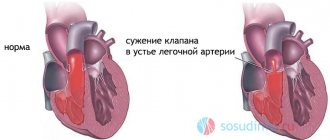
Valve stenosis
With a valve abnormality such as stenosis, the valve for some reason does not open enough to allow a portion of blood to pass into another chamber of the heart.
The symptoms of stenosis are somewhat different from the symptoms of insufficiency. Due to obstructed blood flow, a person feels dizzy, fatigued, and often faints due to poor circulation in the vessels of the brain. But with grade 1 pulmonary valve stenosis, a person does not yet feel such serious symptoms, he only more often feels fatigue.
The valve works increasingly worse if the stenosis is not treated and the advice of the cardiologist is not followed. First comes the compensation stage, when the heart works at double the rate to ensure blood flow. And then the situation gets even worse. The stage of decompensation begins, the right ventricle expands, as there is too much blood in it. And its muscle walls are not able to push this mass of blood through the narrowed walls of the valve.
Severe pulmonary valve stenosis is treated mainly with xenopericardial replacement. That is, extremely quickly. The operation is indicated for those people who have severe right ventricular failure and are at risk of death.
Choosing a treatment method
Treatment depends on the reason for the regurgitation of the pulmonary artery and pulmonary valve. If a person does not have hemodynamic disturbances or changes in the cardiac system, then he does not need special treatment. It is enough for such a patient to be observed by a cardiologist
Important! If cardiac function is impaired due to regurgitation, then there is a need to provide surgical and conservative treatment. The choice of therapeutic tactics depends on the patient’s condition, the presence of contraindications and indications for certain methods.
Isolated stenosis in the pulmonary trunk in the neonatal period
Isolated stenosis, that is, stenosis not associated with other heart diseases, is formed in the intrauterine (neonatal) period of fetal development in the following cases:
- the mother suffered from rubella during pregnancy;
- has diabetes of the 1st or 2nd degree;
- the woman drank alcohol;
- genomic damage;
- substances in the mother’s body such as isotretinoin, used to treat seborrhea, also lead to defects; or hydantoin, a substance used in anti-convulsant medications.
Clinical manifestations of pulmonary valve stenosis in newborns occur in different ways. In mild cases, the defect does not make itself felt and is asymptomatic. And in severe cases, from the first days of life there is a severe lack of blood supply to the tissues and cyanosis.
Results of balloon valvuloplasty in patients with pulmonary valve dysplasia
One of the serious problems when performing balloon valvuloplasty is the presence of pulmonary valve dysplasia. The main criteria for pulmonary valve dysplasia, developed by E. Koretzky et al. and R. Jeffery et al., are: a) angiographically uneven, nodular contours of thickened and flattened valve leaflets; b) hypoplastic pulmonary artery ring; c) absence of post-stenotic expansion of the pulmonary artery trunk.
According to many authors, pulmonary valve dysplasia significantly worsens the results of balloon valvuloplasty. Thus, according to A. Rocchini and M. Beekman, out of 7 cases with pulmonary valve dysplasia, balloon valvuloplasty was successful in only 3 patients. However, according to P. Rao et al. Balloon valvuloplasty was successful in 77% (10 of 13) of patients with pulmonary valve hypoplasia. Thus, the systolic pressure gradient between the right ventricle and the pulmonary artery after balloon valvuloplasty decreased from 77.2 to 26.8 mm Hg, and in the long term (on average after 10 months) it was 34.9 mm Hg.
In 10 successful balloon valvuloplasties, the diameter of the balloon catheter used in relation to the diameter of the valve ring was 1.37±0.22 and was significantly larger than in 3 patients (1.04±0.17) in whom the desired result was not achieved. Based on these data, the authors believe that in patients with pulmonary valve dysplasia it is necessary to use balloon catheters of a larger diameter than in patients without pulmonary valve dysplasia, that is, the ratio of the diameter of the balloon to the diameter of the pulmonary valve ring should be 1.4-1. 5.
According to some authors, the results of balloon valvuloplasty depend on the degree of dysplasia and on the combination of pulmonary valve dysplasia with fusion of the leaflets along the commissures. In this case, the rupture along the commissures becomes the leading link in the mechanism of balloon valvuloplasty for this pathology. N. Muzewe et al. believe that in the presence of echocardiography and angiocardiographic signs of fusion of the leaflets along the commissures, it makes sense to perform balloon valvuloplasty.
According to our data, pulmonary valve dysplasia was detected in 6 (1.13%) of 533 patients. The age of the patients ranged from 22 days to 14 years. The systolic pressure gradient between the right ventricle and the pulmonary artery on average in this group was 124.2±10.2 mmHg before balloon valvuloplasty, after - 38.2±9.3 mmHg, and in the long term (on average after 11.2 months) – 44.2 ± 10.1 mm Hg.
However, in 3 patients in whom a balloon catheter was used, the diameter of which exceeded the diameter of the valve ring by more than 30%, there was a significant decrease in the systolic pressure gradient between the right ventricle and the pulmonary artery (29.2 ± 5.6 mm Hg .) and more durable results in the long term (31.2±6.3 mm Hg), compared with 3 other patients who used balloon catheters whose diameter in relation to the diameter of the valve ring was less than 1, 1 (49.3±10.2 and 56.4±12.1 mmHg, respectively).
Thus, based on the above, we can conclude that for pulmonary valve dysplasia, balloon valvuloplasty is effective if there are signs of commissure fusion and when using a balloon catheter that exceeds the diameter of the pulmonary artery valve ring by 30-50%.
Diagnostics
How does a doctor make a diagnosis, what tests and procedures will he require? The cardiologist, in fact, uses a standard program for examining the heart and its defects. He cannot make a diagnosis based on the patient's complaints alone. He needs to objectify, then specify the problem, find out at what stage the disease is.
The following studies are carried out:
- chest x-ray;
- ECG and echoECG;
- catheterization of cavities;
- lab tests;
- angiopulmonography using a contrast agent.
In addition, the doctor looks for other signs, such as swelling of the neck veins. During auscultation, noises are sometimes heard; The doctor can determine the duration of these noises and can make assumptions about their nature. However, his assumption still needs to be confirmed using the listed procedures. If the pulmonary valve is functioning normally and no abnormal sounds are heard, no procedure is required.
Pregnant women are specially given an ultrasound to find out whether there is a risk of heart pathologies in the fetus.
Diagnostic methods
An important step in the treatment of this disease is its diagnosis. So, it is necessary not only to establish the presence of deficiency, but also to find out what type and severity it has. Diagnostics is carried out in several stages:
- collecting an anamnesis of the disease, recording all complaints related to this problem (swelling, shortness of breath, palpitations, etc.);
- collecting a life history to determine the cause of the disease;
- examination of the patient (the doctor pays attention to the external signs of the disease - cyanosis, acrocyanosis, enlarged abdomen, prominent neck veins, expansion of the heart to the right, swelling of the legs, noise during diastole);
- urine and blood tests to confirm inflammation;
- biochemical blood test for cholesterol, creatinine and other indicators;
- immunological analysis to detect antibodies to possible pathogens of infection leading to heart pathology;
- ECG;
- phonocardiography (analysis of heart murmurs);
- Echocardiography (ultrasound of the heart) is a selective diagnostic method that allows you to accurately determine the presence of a defect. It is also possible to study with a Doppler sensor;
- other methods (chest x-ray, spiral CT, cardiac catheterization, etc.).
ECG for aortic valve insufficiency
A set of diagnostic methods is important in order not only to establish the presence of a disease, but to collect more information about it. After all, treatment can be performed surgically, and when preparing the operation, it is important to take into account all the factors that may affect its success.
How is prosthetic surgery performed?
The operation is prescribed for those individuals who have been diagnosed with subcompensated or decompensated pulmonary valve disease. Judging by the situation, the doctor decides whether to keep the valve or install a new one. Artificial valves exist, both mechanical and biological. However, the service life of the biological one is only 15 years, then the operation is required again. That’s why young people are immediately given a mechanical one.
For those people who have suffered a stroke or myocardial infarction, surgery is contraindicated. Diabetics are also prohibited from performing such a serious operation.
Before the operation, the patient is prohibited from eating food and is stopped taking all medications 12 hours before the operation. The day before, the person is offered to drink a sedative so that he does not set himself up for bad thoughts and is not afraid. After all, the operation is performed on an open heart, and at this time its function is performed by a heart-lung machine. But the risk, thanks to honed skills and teamwork of specialists, is minimal.
After surgery, the patient must undergo a rehabilitation course. The course program usually includes physical therapy and special breathing exercises.
Choosing a treatment method
Treatment depends on the reason for the regurgitation of the pulmonary artery and pulmonary valve. If a person does not have hemodynamic disturbances or changes in the cardiac system, then he does not need special treatment. It is enough for such a patient to be observed by a cardiologist
Treatment
Important! If cardiac function is impaired due to regurgitation, then there is a need to provide surgical and conservative treatment. The choice of therapeutic tactics depends on the patient’s condition, the presence of contraindications and indications for certain methods.
{SOURCE}
Prevention of the development of defects
The best prevention of valve dysfunction at all times is to maintain a healthy lifestyle. No vitamin supplements or “golden formulas” of youth will help maintain health if a person smokes from a young age and does not follow a sleep-wake schedule.
The human heart is a very vulnerable organ. Smoking and alcohol cause irreparable harm to him. And another factor for heart health is that humans are designed to move. At any age, he should play sports, but in moderation. Heavy loads for the sake of results are also harmful.
Women during pregnancy should avoid various mutagenic factors and not take medications without consulting their doctor. Many drugs in the prenatal period can lead to pulmonary valve defects.
normal blood pressure, function and disease
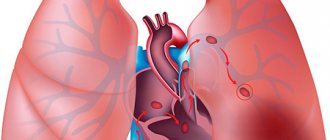
The pulmonary artery is a unique vessel belonging to the pulmonary circulation. This is the only artery in the body that carries venous blood. This large paired vessel is entrusted with serious functions, on the fulfillment of which not only human health, but also human life depends.
Structure of the pulmonary artery
The location of the pulmonary artery is the central part of the chest. This blood vessel consists of two large branches and several small ones, branching into a capillary network. The average diameter of large branches is 25 mm.
The PA is connected to the right ventricle. Branching from it, the artery is located next to other large vessels. Dividing, the branches of the pulmonary artery enter the lungs, one into the right, the other into the left. At the base of the PA there is a valve that ensures timely blood flow from the right ventricle.
Location of aircraft branches:
- The left branch is a continuation of the pulmonary trunk. It is located in front of the aorta and the descending part of the left bronchus.
- The right branch is longer than the left one. On one side of the vessel is the superior vena cava and the ascending aorta. On the other is the main right bronchus.
Conclusion
Defects of the mitral and aortic valves are more common than those of the pulmonary valve. Valvular insufficiency of the left side of the heart usually follows after the left side fails at the stage of decompensation; and then the right side of the heart “breaks.”
Infectious endocarditis is also considered a common cause of pulmonary valve defects. Older people are advised to prevent endocarditis and consult a cardiologist more often for a routine examination.
However, if it is nevertheless diagnosed after a detailed study, then the patient needs to completely reconsider his life.
Treatment approaches
Depending on the stage of the disease (1, 2, 3, etc.), as well as the causes of its occurrence, treatment can be either medicinal or surgical. First of all, they eliminate the causes of insufficiency (get rid of infection, eliminate accumulated fluid in the lungs, etc.). Then, if necessary, medications are prescribed:
- angiotensin-converting enzyme inhibitors (they normalize blood pressure, eliminate arrhythmia, normalize heart function, dilate blood vessels);
- angiotensin 2 receptor antagonists (used if the former do not work or are contraindicated);
- nitrates (for example, nitroglycerin, which dilates arteries and veins, thereby reducing pressure in them);
- diuretics (eliminate accumulated fluid in the body, relieve swelling).
If it is necessary to eliminate special symptoms of insufficiency (for example, arrhythmia, ventricular failure), medications are also prescribed to treat them.
If surgical intervention is necessary (as a rule, it is required if there are severe problems with the valve, which entail serious hemodynamic disturbances), one of the following operations is performed:
- plastic surgery (reducing the thickness of the pulmonary artery trunk);
- valve replacement (using natural or artificial substitutes);
- heart and lung transplantation (if the heart already has serious disorders and the lungs have persistent high pulmonary hypertension).
Surgery is also needed if, in addition to pulmonary valve disease, there are other problems (for example, atrial or ventricular septal defect). Then all problems are eliminated at once.
After the operation, treatment does not end. It is necessary to monitor the patient. If a mechanical prosthesis is installed, indirect anticoagulants are constantly taken, but if a biological one is installed, for 1-3 months. And only after plastic surgery, drug therapy is not required.
Pulmonary valve - Wikipedia
Material from Wikipedia - the free encyclopedia
Pulmonary valve
Pulmonary valve
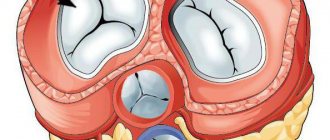
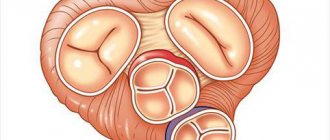
(lat. valva trunci pulmonalis) - one of the valves of the human heart, or other warm-blooded animals, located on the border of the right ventricle and the pulmonary trunk (which at the level of the 4th thoracic vertebra is divided into the left and right pulmonary arteries), preventing the reverse flow of blood from pulmonary trunk into the right ventricle in diastole. In humans, the valve has three leaflets that open towards the pulmonary trunk. The semilunar valves, closing, block the opening connecting the pulmonary trunk and the right ventricle. The leaflets are attached to the fibrous ring, which forms an opening between the pulmonary trunk and the right ventricle.
In the literature also called the pulmonary valve
or
pulmonary valve
.
The pulmonary valve is separated from the fibrous framework of the heart by the muscular septum of the right ventricular outflow tract. Its semilunar base rests on the myocardium of the right ventricular outflow tract.
The valve consists of three sinuses, three semilunar leaflets, extending with their bases from the fibrous ring of the base of the valve.
Doors[edit | edit code]
Lunar valves
(anterior, left and right) originate from the medial edge of the annulus fibrosus of the valve. Their proximal edges continue laterally in the form of sinuses, and the free edges protrude into the pulmonary trunk.[1]
The thickened fibrous part of the central zone of closure of each leaflet is called the nodes of Morgagni
.
The left leaflet directly borders the muscle tissue of the outflow tract of the right ventricle, its septum and the upper part of the supraventricular crest. The right leaflet is also adjacent to the myocardium of the right ventricular outflow tract.
The leaflets have a three-layer structure and consist of the ventricular, median and sinus layers. Their thickness is maximum at the fibrous ring and minimum at the dome. The blood supply to the valves is provided by a large number of arterioles, veins and capillaries located at their base.
Sinuses[edit | edit code]
Sinuses
The pulmonary valve is named according to the names of the leaflets, the anatomical continuation of which is: anterior, left and right.
The wall of the sinuses at the sinotobular junction has a structure similar to the wall of the pulmonary trunk, with a well-defined middle layer consisting of smooth myocytes surrounded by elastin and collagen fibers. Towards the fibrous ring, the wall becomes thinner, the number of elastin fibers and myocytes decreases, collagen fibers increase, and the wall of the sinuses takes on the appearance of a fibrous cord.
Sinotobular junction[edit | edit code]
Sinotobular junction
(arched ring or arched ridge) - connection between the sinuses and the pulmonary trunk.
Fibrous ring[edit | edit code]
Valve base fibrous ring
has a triangular cross-section and consists mainly of collagen structures and an elastic membrane (along its ventricular surface). It begins with a bifurcation of the fibrous cord of the sinus, one part of which creates the sinus wall of the ring (further passing on to the leaflet), and the other part forms the base of the triangle of the fibrous ring and entwines cardiomyocytes. The tissues that make up the middle part of the fibrous ring pass into the valve and form its middle layer.
Commissary[edit | edit code]
Commissura
- line of contact of adjacent sashes.
Commissural rods
- the attachment points of the commissures on the inner surface of the valve consist of three sections: the arched section, which is a continuation of the arched ridges and has their structure, and the fibrous section, consisting of uncrimped collagen bundles, braided with highly crimped thin collagen fibers, similar in structure to the fibrous ring , and the section of transition from the first to the second.
- ↑ Surgical anatomy of the valve apparatus of the aorta and pulmonary artery in the light of the treatment of heart defects / Goncharov O. G. // Vestn. hir. them. I. I. Grekova. - 1956. - No. 7. - P. 74-79.
- Prives M. G., Lysenkov N. K.
Human anatomy. — 11th revised and expanded. - Hippocrates. — 389 p. — 5000 copies. — ISBN 5-8232-0192-3.
Pulmonary regurgitation: pathophysiology, causes, symptoms, treatment
Pulmonary regurgitation or pulmonary valve incompetence occurs as a result of one of three main pathological processes: dilatation of the pulmonary valve annulus, an acquired change in the morphology of the pulmonary valve leaflet, or a congenital absence or malformation of the valve. PR leads to right ventricular volume overload, which subsequently leads to right ventricular enlargement and dysfunction. Over time, PR will lead to tricuspid regurgitation.
Significant pulmonary or pulmonary regurgitation occurs in different ways as a complication of different conditions.
The most common causes of a leaky pulmonary valve are pulmonary hypertension or congenital heart disease (most commonly tetralogy of Fallot).
Less common causes of LR include the following:
- Infective endocarditis
- Carcinoid syndrome
- Complications after surgical repair of tetralogy of Fallot
- Rheumatic fever
Primary pulmonary hypertension occurs in approximately 1 in 500,000 cases. This diagnosis can only be made after all other causes have been excluded. Primary causes include iatrogenic, infective endocarditis, systemic (carcinoid disease), immune-mediated (rheumatic heart disease), and congenital heart disease.
Secondary pulmonary hypertension (multiple causes) is the most common cause of pulmonary regurgitation in adults. Secondary or functional PR occurs in patients with a normal pulmonary valve who have severe pulmonary arterial hypertension and/or pulmonary artery dilatation.
Tetralogy of Fallot, especially when there is congenital absence of the pulmonary valve or in the postoperative period after surgical repair of this condition (eg, pulmonary valvotomy), usually causes significant PR.
In rare cases, infective endocarditis leads to significant pulmonary regurgitation. This may occur in an IV/injection drug user or in a person with an atrial septal defect and a large left-to-right intracardiac shunt.
In rheumatic heart disease leading to significant PR, the pulmonary valve is affected after the mitral, aortic and tricuspid valves are affected.
Medications
Drugs that act through serotonergic pathways may result in significant PR (eg, methysergide, pergolide, fenfluramine).
Disorders that dilate the pulmonary valve annulus to create valvular incompetence are the most common cause of PR and include primary or secondary pulmonary hypertension, pulmonary trunk dilatation in Marfan syndrome or Takayasu's artery, and idiopathic causes.
Acquired conditions that alter the morphology of the pulmonary valve include the following:
- Rheumatic heart disease: In most cases, the other valves (ie, mitral, aortic, tricuspid) are also significantly affected.
- Swan-Ganz catheter injury: This cause is uncommon, but can occur if the tip of the catheter is pulled through the pulmonary valve with the balloon inflated.
- Complications associated with dilatation of a therapeutic balloon catheter of a stenotic pulmonary valve (eg, pulmonary balloon valvuloplasty): such complications are not uncommon; however, in most cases, the degree of regurgitation is clinically insignificant, making balloon catheter pulmonary valve dilatation a safe and effective treatment for moderate to severe pulmonary stenosis in adults and children.
- Complications of surgical repair of pulmonary stenosis or congenital heart disease such as tetralogy of Fallot
- Carcinoid heart disease: In 60% of patients whose carcinoid heart disease has metastasized to the liver, the heart is affected, most often manifesting as valvular disease. In a series of 74 patients, the pulmonary valve was involved in 88%. Of these, 49% had significant pulmonary stenosis, and 81% had significant PR.
These include complete absence of the pulmonary valve and valvular abnormalities (eg, fenestrations).
Symptoms
Pulmonary or pulmonary regurgitation is rarely clinically significant. There are usually no early symptoms that are noticed by the patient. Eventually, the lower right chamber of the heart may become enlarged and dysfunctional due to a valve problem or pulmonary hypertension. Rarely, it may progress to heart failure.
Symptoms of right-sided heart failure may occur when the severity and duration of regurgitation causes enlargement and decompensation of the right ventricle. Shortness of breath on exertion is the most common complaint.
Mild fatigue, dizziness, peripheral edema, chest pain, palpitations, and outright syncope may occur in patients with any cause of right-sided heart failure and do little to elucidate their etiology.
Patients who experience these symptoms may attribute them to poor fitness or anxiety, delaying evaluation until their condition worsens. In later manifestations of right-sided heart failure, abdominal distension secondary to ascites, right upper quadrant pain secondary to liver distension, and early satiety may be present.
Other symptoms characteristic of the underlying disease causing LR may occur.
Such disease processes include connective tissue disease, infective endocarditis, carcinoid heart disease, rheumatic heart disease, and primary or secondary pulmonary hypertension.
For example, hemoptysis is generally not associated with PR per se, but in severe pulmonary hypertension causing PR, it may result from pulmonary arteriole rupture and hemorrhage and/or parenchymal inflammation.
Physical examination
Cardiac testing for pulmonary or pulmonary regurgitation depends on the severity and cause of the disease.
Jugular venous pressure is usually elevated. A-wave enhancement is often observed, but this may be less obvious when significant tricuspid regurgitation with a dominant B-wave is also present.
When right ventricular enlargement is present, there is usually a palpable impulse (rising or rising) at the left lower sternal border. Palpable pulmonary artery pulsation on the left upper sternum may be present when the pulmonary artery is significantly dilated.
With significant pulmonary hypertension, the pulmonary valve closes.
Signs of pulmonary regurgitation that may be detected on clinical examination include a unique murmur. In the right ventricle, murmurs begin in early diastole and are most noticeable in the left, second, and third spaces. The intensity of the noise increases during inspiration.
The pulmonary component of the second heart sound (P2) is not heard in the absence of a pulmonary valve, whether congenital or secondary to surgical resection.
The RV associated with pulmonary hypertension has a higher-pitched and blowing decrescendo murmur with an enhanced P2 component of the second heart sound; as right ventricular end-diastolic volume increases, ejection time increases, P2 is delayed, and S2 increases.
Regurgitant low-pressure flow through the pulmonary valve, occurring at normal pulmonary artery pressure, is heard as a brief, early diastolic murmur at the upper left sternal border.
This is made louder by squatting or inhaling and softer by Valsalva maneuvers or exhaling.
S3 or S4 may be noted at the left middle and lower borders of the sternum due to the presence of RV hypertrophy or insufficiency and worsens with inspiration.
With more significant PR, there may be a systolic ejection murmur heard in the left upper sternum due to increased RV stroke volume. Increased right ventricular impulse may be present.
The Graham Steele murmur of pulmonary hypertension is a high-frequency, early diastolic decrescendo murmur noted in the left upper left mid chest and is the result of high-velocity burp flow through an incompetent pulmonary valve.
Regurgitation sounds may be present throughout diastole because there is a pressure gradient from the pulmonary artery to the RV during this period of time.
As a rule, the murmur occurs in severe pulmonary hypertension, when the systolic pressure in the pulmonary artery is more than 60 mmHg. The quality of this high early decrescendo diastolic murmur is identical to that of aortic regurgitation.
However, there are no peripheral manifestations of aortic insufficiency. Associated findings of tricuspid regurgitation are common.
Treatment
Pulmonary or pulmonary regurgitation is rarely severe enough to require special treatment because the right ventricle usually adapts readily to low pressure volume overload. High-pressure volume overload leads to right-sided cardiac strain and ultimately to heart failure.
Treatment for LR usually focuses on the underlying cause that created the valve problem (eg, pulmonary hypertension). The underlying etiology causing severe RF, congenital or acquired, must be treated to prevent or reverse right-sided heart strain and failure, which may further complicate the clinical picture
If pulmonary hypertension is identified with LR, determining the etiology is important to promptly initiate appropriate therapy.
For example, primary pulmonary hypertension, secondary pulmonary hypertension due to thromboembolism, severe mitral stenosis, and pulmonary carcinomatosis may present as severe pulmonary hypertension with PR.
A discussion of therapeutic interventions for pulmonary hypertension by etiology is beyond the scope of this article.
The need for surgical pulmonary valve replacement is very rare.
The requirements for transfer are the same as for heart failure.
Consider consultation with cardiologists for patients with right-sided heart failure in the presence of severe pulmonary regurgitation.
No aspect of the medical management of heart failure is unique to pulmonary or pulmonary regurgitation, and a discussion of the management of right-sided heart failure is beyond the scope of this article.
In general, approaches similar to those used in the treatment of patients with left congestive heart failure may be useful.
In some circumstances, such as in patients with pulmonary hypertension, vasodilator therapy should be very carefully considered and tested.
Aspects of inpatient care are primarily governed by the treatment indicated for the specific disorder causing LD. As mentioned earlier, if heart failure caused or aggravated by LR is present, conventional heart failure treatment is used.
As noted previously, recommendations for the prevention of infective endocarditis do not support the need for antibiotic prophylaxis for pulmonary regurgitation for other structurally normal lung valves, especially if a diastolic murmur is not heard.
However, PR in congenital heart disease, acquired valvular dysfunction as in rheumatic heart disease, complex cyanotic heart disease, prosthetic valves, and preexisting bacterial endocarditis include moderate to high-risk conditions that require antibiotic prophylaxis.
Surgical therapy
When right-sided heart failure due to pulmonary or pulmonary regurgitation from an abnormal pulmonary valve cannot be improved by medical treatment, appropriate options include surgical reconstruction or pulmonary valve replacement, preferably with a bioprosthetic valve.
Bioprosthetic valves, which last up to 15 years after implantation, are generally preferred over mechanical prosthetic valves.
Ongoing technological advances include the investigation of new valves for use in larger, non-conducting outflow tracts (compared to fixed-size conduits) and the addition of a hybrid surgical and transcatheter approach to pulmonary valve implantation.
Source: https://cardio-bolezni.ru/legochnaya-regurgitatsiya-patofiziologiya-prichiny-simptomy-lechenie/