Pathophysiology
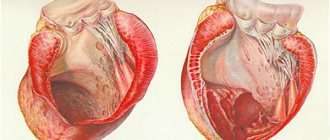
The development of a true left ventricular aneurysm occurs in two main phases: early dilatation and late remodeling.
True left ventricular aneurysm (at least in 88%) usually develops after transmural myocardial infarction due to thrombosis of the anterior interventricular branch (LAD) and insufficient development of collateral circulation. The early dilatation phase begins within 48 hours of myocardial infarction in 50% of patients. In other patients, aneurysm formation occurs within 2 weeks after myocardial infarction. Within a few days, the inner surface of the developing aneurysm loses its trabecularity and becomes smooth, and a left ventricular thrombus forms in 50% of patients.
On days 2-3, leukocytes migrate into the infarction area, which leads to lysis of necrotic myocytes on days 5-10 after the infarction. As a result of the destruction of collagen and myocytes, the strength of the myocardial wall by this time is significantly reduced and myocardial rupture is possible. Preservation of hibernating myocardium in the peri-infarction zone is a prerequisite for preventing the development of a true left ventricular aneurysm.
The loss of systolic activity in the infarcted zone while maintaining contractility in the surrounding myocardium causes systolic protrusion and thinning of the myocardial region. According to Laplace's law (T = Pr/2h), with constant pressure in the left ventricle (P), an increase in the radius of curvature (r) and a decrease in the wall thickness of the left ventricle (h), wall tension (T) increases in the infarct zone.
Compared to normal, damaged (infarcted) myocardium is more plastic and susceptible to deformation. Thus, increased systolic and diastolic stress on the left ventricular wall leads to progressive expansion of the infarcted area until scar formation reduces the plasticity of the aneurysmal area.
Due to an increase in diastolic tension and preload, as well as an increase in the level of endogenous catecholamines, unaffected areas of the myocardium are forced to develop increased contraction, which leads to myocardial hypertrophy. This in turn causes an increase in oxygen consumption by healthy parts of the myocardium and the left ventricle as a whole.
In addition to this, when an aneurysm forms, a decrease in stroke volume occurs, since part of it is ejected not into the aorta, but into the aneurysm. Net mechanical efficiency of the left ventricle (external stroke work minus myocardial oxygen consumption) decreases, decreasing external stroke work and further increasing myocardial oxygen consumption.
Left ventricular aneurysm causes not only systolic but also diastolic ventricular dysfunction. Diastolic dysfunction results from increased stiffness of the fibrous aneurysmal wall, which impedes diastolic filling and increases left ventricular end-diastolic pressure.
It begins 2-4 weeks after myocardial infarction, when highly vascularized granulation tissue appears, which is replaced after 6-8 weeks by fibrous fibrous tissue. Arrhythmias, such as ventricular tachycardia, can develop at any time during the development of a ventricular aneurysm, since re-entry microfoci develop in the peri-infarction zone.
Nitrate therapy for only 2 weeks after a heart attack does not prevent aneurysm formation. The role of angiotensin-converting enzyme inhibitors is still unclear since they suppress the development of ventricular hypertrophy. Myocardial revascularization, improving coronary perfusion and the movement of fibroblasts into the infarcted area of the myocardium, is a good preventive factor for the development of left ventricular aneurysms. The use of steroids, on the contrary, can increase the likelihood of aneurysm formation.
Despite significant advances in the molecular, biomechanical, genetic, neurohumoral and pharmacological fields, understanding the structure and function of the cardiomyocyte, one should not, however, forget the fundamental fact that highly specialized cells can function effectively only surrounded by a collagen skeleton, which not only has them relative to each other, but also connects cardiomyocytes.
Thus, a set of morphological units is created that is capable of generating force within a certain structural location - the muscle band, and of transforming simple shortening of contractile units into mechanically effective contraction and relaxation of the spiral structure of the entire myocardium of the left ventricle of the heart.
Any improvement in myocyte function in the absence of an adequate collagen scaffold, and, accordingly, improvement in systolic and diastolic function of the entire heart, is impossible. There is general agreement that collagen plays an important role in maintaining cardiac size and shape and in post-infarction remodeling.
It makes up 1% to 4% of all cardiac proteins. Collagen fibers are supramolecular formations that consist of collagen molecules arranged in a zigzag and criss-cross pattern to increase strength. The extracellular matrix is represented by a viscoelastic environment of type I and III collagen, which binds myocytes, determines the interaction between myofilaments and maintains the relationship between capillaries and myocytes.
The collagen scaffold consists of a network of myofibrils and intercellular “spacers” that connect neighboring myocytes, which allows the myofibrils to optimize the development of muscle force, distribute it within the ventricular walls and prevent deformation of the sarcomeres. Inside the above-described structure, the intercellular space is filled with proteoglycans.
Myocardial remodeling represents the adaptive response of the heart to prolonged exposure to physiological and pathogenic factors. At the same time, the structure of myocytes and extracellular matrix changes. Reduction of myocardial collagen cross-linking has been shown in animal models. Since the structural support provided by fibrillar intracellular collagen is an important determinant of myocyte shape and mass distribution, as well as a component of the conversion of myocyte contraction into total cardiac output, its loss, due to the degradation of mature collagen with its replacement by newly synthesized one with a reduced amount cross-links, can directly affect systolic dysfunction and dilation of the heart cavities.
Changes in the extracellular matrix are necessary for the formation of a new structure of the heart chambers. The development of fibrosis, namely an increase in the amount of extracellular matrix proteins - collagen types I and III, must necessarily be preceded by destruction of the collagen network. Type I collagen takes part in the formation of the extracellular matrix during the late remodeling phase, while type III collagen takes part in the early expansion phase.
Any change in the extracellular matrix essentially means an imbalance between the rates of protein synthesis and breakdown. Being organized in a fibrillar form, extracellular collagen is extremely resistant to destruction by proteases, with the exception of specific collagenases - matrix metalloproteinases (MMPs), the main sources of which in the heart are fibroblasts.
Changes in the structure of collagen and its distribution during the remodeling process depend on the regulation of MMPs at three levels: transcription, activation and inhibition by tissue inhibitors of metalloproteinases (TIMMPs). TIMMPs are low molecular weight proteins that have high affinity for the catalytic domain of MMPs.
Thus, TIMMPs neutralize collagen degradation. The genes encoding MMPs and TIMMPs are colocated and coexpressed. This MMP induction/activation system has been found in the sarcolemma of myocytes. It does not work correctly in patients with ischemic cardiomyopathy. Collagenases can be activated through a variety of mechanisms, including tumor necrosis factor-alpha (TNF-alpha), free radicals, insulin-like growth factor-1, transforming growth factor-1, which stimulates fibroblast proliferation, catecholamines—all factors that occur during ischemia.
One of the endogenous activators of MMP may be chymase (the only enzyme in myocardial tissue that converts angiotensin I to angiotensin II), an increased level of which was determined during conditions of pressure or volume overload. One of the donors of sulfhydryl groups, oxidized glutathione, which occurs in the zone of myocardial ischemia, also activates latent collagenases and causes rapid destruction of collagen bridges in the “stunned” myocardium and in the infarction zone within 2-3 hours after arterial occlusion, when inflammatory disease has not yet developed. infiltrate.
We invite you to read: Subendocardial myocardial infarction - what is it?
Local synthesis of aldosterone by myofibroblasts causes autocrine stimulation of the renin-angiotensin-aldosterone system. Together with angiotensin II and atrial natriuric factor, aldosterone stimulates the translation of m-RNA of collagen types I and III.
Another point requiring close attention is the possible reversibility of extracellular matrix remodeling. Recent studies have shown that long-term use of circulatory support systems reverses contractile dysfunction and influences gene expression in end-stage heart failure.
Improvements in echocardiographic parameters were also noted. Thus, it becomes clear that cessation of the action of the factor, the load, that triggers pathological remodeling and the development of heart failure, can lead to the reverse development of structural and architectural changes. Since the basis for activation and modulation of intracellular chain reactions is mechanical stress, reducing the latter, respectively, achieving control over the expression and activity of MMPs, is possible only with the use of volume-reducing surgery and modification of ventricular geometry.
What is myocardial hypertrophy
This autosomal dominant disease is characterized by hereditary traits of gene mutation and affects the heart. It is characterized by an increase in the thickness of the walls of the ventricles. Hypertrophic cardiomyopathy (HCM) has a classification code according to ICD 10 No. 142. The disease is often asymmetric, with the left ventricle of the heart more susceptible to damage. This happens:
- chaotic arrangement of muscle fibers;
- damage to small coronary vessels;
- formation of areas of fibrosis;
- obstruction of blood flow - an obstacle to the ejection of blood from the atrium due to displacement of the mitral valve.
With heavy loads on the myocardium caused by diseases, sports, or bad habits, the body’s protective reaction begins. The heart needs to cope with increased work volumes without increasing the load per unit of mass. Compensation begins to occur:
- increased protein production;
- hyperplasia – increase in the number of cells;
- increase in myocardial muscle mass;
- wall thickening.
Pathological myocardial hypertrophy
With prolonged work of the myocardium under loads that are constantly increased, a pathological form of HCM occurs. A hypertrophied heart is forced to adapt to new conditions. Myocardial thickening occurs at a rapid pace. In this situation:
- growth of capillaries and nerves lags behind;
- blood supply is disrupted;
- the influence of nervous tissue on metabolic processes changes;
- myocardial structures wear out;
- the ratio of myocardial sizes changes;
- systolic and diastolic dysfunction occurs;
- repolarization is disrupted.
Myocardial hypertrophy in athletes
Abnormal development of the myocardium—hypertrophy—occurs unnoticed in athletes. During high physical activity, the heart pumps large volumes of blood, and the muscles, adapting to such conditions, increase in size. Hypertrophy becomes dangerous, causing stroke, heart attack, sudden cardiac arrest, in the absence of complaints and symptoms. You should not suddenly stop training to avoid complications.
Sports myocardial hypertrophy has 3 types:
- eccentric - muscles change proportionally - typical for dynamic activities - swimming, skiing, long-distance running;
- concentric hypertrophy - the ventricular cavity remains unchanged, the myocardium increases - observed in gaming and static types;
- mixed - inherent in activities with the simultaneous use of immobility and dynamics - rowing, cycling, skating.
Myocardial hypertrophy in a child
It is possible that myocardial pathologies may appear from the moment of birth. Diagnosis at this age is difficult. Hypertrophic changes in the myocardium are often observed during adolescence, when cardiomyocyte cells actively grow. Thickening of the anterior and posterior walls occurs until the age of 18, then stops. Ventricular hypertrophy in a child is not considered a separate disease - it is a manifestation of numerous ailments. Children with HCM often have:
- heart disease;
- myocardial dystrophy;
- hypertension;
- angina pectoris.
Natural course
Relatively new studies have found 47-70% 5-year survival rates for patients with left ventricular aneurysms. Causes of death include arrhythmia in 44%, cardiac arrest in 33%, myocardial infarction in 11% and non-cardiac causes in 22%.
Factors influencing survival are age, degree of coronary disease, duration of angina before previous infarction, ischemic mitral regurgitation, ventricular arrhythmias, aneurysm size, contractile function of viable myocardium, left ventricular cardiopulmonary dysfunction. Early aneurysm development within 48 hours of infarction also reduces survival.
The risk of thromboembolism for patients with aneurysms is low (0.35% of patient-years), and chronic use of anticoagulants is generally not recommended. However, 19% of patients with echocardiographically visible thrombi after myocardial infarction experienced episodes of thromboembolism. Atrial fibrillation and large aneurysm size are additional risk factors for thromboembolism.
Rupture of chronic left ventricular pseudoaneurysms is less common than might be expected. Rupture of left ventricular pseudoaneurysms may be most likely in the acute phase of myocardial infarction or when they are large. Ventricular pseudoaneurysms tend to behave similarly to true aneurysms.
Clinical signs
Signs of left ventricular hypertrophy are absent for a long time. Symptoms appear only when a person cannot compensate for the resulting changes in blood circulation. Concentric hypertrophy of the left ventricular myocardium can be manifested by the following symptoms:
- dizziness;
- pain in the heart;
- shortness of breath;
- swelling of the lower extremities;
- sleep disturbance;
- decreased ability to work;
- weakness;
- feeling of a sinking heart;
- fainting;
- lability of blood pressure;
- heart rhythm disturbances such as atrial fibrillation or extrasystole.
Most of these patients experience pain in the heart area similar to angina pectoris. Increased blood pressure is common. A typical manifestation of myocardial hypertrophy is shortness of breath. In the early stages it bothers you when working, and then appears at rest. In severe cases, cardiac asthma develops. Many patients are bothered by periodic attacks of suffocation.
Acrocyanosis (blue discoloration of fingers, nose, lips) is possible. All these symptoms are caused by the underlying disease, which led to left ventricular hypertrophy. If the cause is hypertrophic cardiomyopathy, then the outcome of such a pathology can be non-violent death due to sudden cardiac arrest.
Hypokinesia as the main syndrome of parkinsonism
Hypokinetic syndrome manifests itself in parkinsonism both in the limbs and in the body.
These are all the above-mentioned types of motor function disorders against the background of plastic hypertonia of the muscles, including paradoxical movements, pallidal tremor, micrographic handwriting, the phenomena of “wax doll pose” and “cogwheel”, when walking – body drifts to the side with falls, Parkinson’s marking in place, acheirokinesis.
A typical pose is with the head and body tilted forward with the chin touching the chest, with the arms pressed to the body, bent at the wrist and elbow joints, with flexion of the fingers at the metacarpophalangeal joints and extension at the interphalangeal joints, with the first finger in opposition to the rest of the fingers of the hand, with the legs , slightly abducted and half-bent at the knees.
Hypertonicity of the neck forces the patient to turn around when called with the whole body or turn the eyes as much as possible, while the head remains motionless.
The motor skills of the muscles that ensure lively facial expressions are also disordered, hence the mask-like appearance of the face (hypomimia) with an almost unblinking gaze, the monotony of a quiet voice due to difficulties with articulation of speech (hypophonia).
A feature of hypokinesia in parkinsonism (in addition to the preservation of muscle strength) is its combination with a high degree of muscle rigidity.
In contrast to spasticity, rigidity manifests itself:
- a positive “lead tube” symptom (uniformity of muscle wax-like resistance, as when bending a lead tube) - spastic muscles respond with the phenomenon of recoil and “jackknife”;
- the invariance of tendon reflexes - with spasticity they increase.
An illustration of rigidity is the “cogwheel” symptom, which is not characteristic of spasticity.
The similarity of movement disorders makes patients with parkinsonism similar to each other, differing only in the expression of their gaze.
The deformity sequence of fetal akinesia includes the type of akinesia that causes a combination of pathologies in the womb as the baby develops. Examples of such symptoms:
- Various contractures;
- Facial abnormalities;
- Intrauterine growth restriction;
- Hypoplasia (underdevelopment) of the lungs.
Approximately 30% of children with fetal akinesia deformity sequence are stillborn. Others cannot live long due to problems associated with pulmonary hypoplasia.
Development mechanism
Moderate left ventricular hypertrophy is very often asymptomatic and in most cases is discovered incidentally after electrocardiography.
How to treat left ventricular hypertrophy depends on the degree of complexity of the course and the characteristics of the initial disease against which this pathological syndrome arose.
The etiological investigation initiated revealed no cardiac history or similar cases. Echocardiography was normal in both parents and twins. The child's karyotype was normal. A series of biological tests were performed and normalized: blood hemoglobin, determination of total carnitine and free carnitine in the blood, determination of urine catecholamines, analysis of glucosidase activity and analysis of creatine kinase. Anatomic pathological examination of the muscle biopsy was also normal.
During the course, there was a clear clinical improvement with regression of cardiac murmur and adequate weight gain. The decision was then made to stop the study and monitor the child, echocardiographic examination at 1, 3 and 6 months was without abnormalities with strictly normal growth.
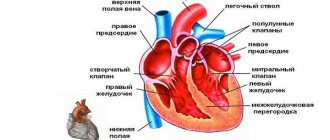
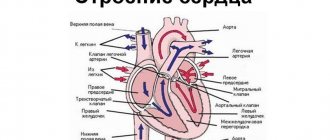
In the absence of physiological disorders, the heart performs the so-called pump function, pushing blood into the aorta with enormous force. After which the blood flows to all organs. At the moment of relaxation of the left ventricle, a portion of blood flows from the left atrium. The process of blood flow is continuous, and its quantity is sufficient to ensure complete gas exchange throughout the body.
After a review of the literature, the etiology used for this short-term neonatal cardiac hypertrophy was a secondary reaction to the administration of corticosteroids to accelerate lung maturation. A potential effect of maternal catecholin excretion during toxicosis of pregnancy cannot be ruled out.
Neonatal hypertrophic cardiomyopathy is a rare entity described in the literature as isolated clinical cases defined by asymmetric left ventricular hypertrophy, predominantly septal with normal systolic function. This entity often presents with a well-tolerated clinical form of rapid regression, in contrast to forms associated with maternal diabetes, where adrenal restitution is sometimes performed after one year. Kalid et al. Reported 3 cases of transient hypertrophic cardiomyopathy in neonates whose mothers received repeated doses of antenatal corticosteroids, the authors believed that the onset of ventricular hypertrophy actually depends on the dose and duration of maternal corticosteroid injection before pregnancy. However, given our observation It is believed that even low doses can cause this pathology, since the mother received only one drug of betamethasone, and the association with stress conditions with the release of catecholamines could potentiate the effect of corticosteroids on the cardiac muscle and promote hypertrophy of cardiac muscle cells.
When pathological disorders of the cardiovascular system occur, the performance of this function becomes significantly more difficult, which leads to excessive energy costs. Increased exercise helps to increase the muscle mass of the heart. This happens in almost the same way as when muscle mass increases after intense physical activity in the gym. And if in the case of this muscle group this is a positive result, then for the heart muscles it is a pathologically dangerous condition. The fact is that the vessels supplying the heart do not have time to grow behind the muscle mass, as a result of which there is insufficient blood supply to the hypertrophied tissue. This, in turn, is the cause of various ischemic changes in the myocardium.
The low weight of the affected child is likely another factor. All of these indices may suggest that it is a multifactorial entity. Others believe that this transcription favors the expression of myosin heavy chain. The recurrence of this entity in often low birth weight preterm infants may provide another indication of the molecular mechanisms involved.
Its rapid regression should resemble one of the metabolic causes, particularly maternal diabetes or an indirect iatrogenic cause, including corticosteroids. Comprehensive etiological assessment is still necessary, but it is expensive and slow, and consensus on a minimum balance established by learned societies may improve and facilitate management.
Return to contents
Diagnostics
The electrocardiogram shows a Q wave and ST segment elevation. A chest x-ray may reveal an enlarged cardiac shadow, which is not pathognomonic of a left ventricular aneurysm. Left ventriculography was until recently the gold standard for diagnosing left ventricular aneurysm.
Two-dimensional echocardiography is also a sensitive method for diagnosing aneurysms, allowing not only to detect the aneurysm itself, but also the presence of a thrombus in it, and also to calculate local and global ejection fraction by segment. Stress echocardiography can additionally determine the presence and volume of hibernating areas of the myocardium.
The essence of the problem
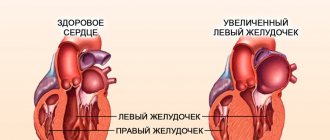
Hypertrophy of the left ventricle of the heart is often a consequence of overload or malfunction of the valve apparatus. This is the main diagnostic sign of hypertrophic cardiomyopathy. The left ventricle of the heart is a cavity, a muscular formation that is capable of contracting and pushing blood. This chamber begins the systemic circulation.
There are several types of hypertrophy: eccentric, concentric and with obstruction. Each form has its own characteristics. Eccentric left ventricular hypertrophy is most often formed due to insufficiency of the valve located between the left parts of the heart. Its development is based on an excess of normal blood volume in this part of the heart.
The weight of the left ventricle increases and it stretches. Such changes negatively affect heart contractions. High load leads to decreased cardiac output. The concentric form of LVH is different in that the blood is thrown back, and the myocardium requires more force to push it into the aortic lumen. This is accompanied by thickening of the walls of the heart chamber. Sometimes a decrease in the ventricular cavity is observed.
Risk factors for akinesia
Parkinson's disease is a disease in which there is a decrease in the production of dopamine in the brain, which affects a person's ability to control their muscles.
Drug-induced parkinsonism occurs when a person takes too many drugs that inhibit dopamine.
Progressive supranuclear palsy is a condition that gradually damages the brain, initially affecting balance while walking.
Hormones – Hypothyroidism or low thyroid hormone levels can lead to akinesia.
In people with Parkinson's disease, men are more likely to experience akinesia than women.
Other risk factors:
- bradykinesia or slow muscle movement;
- Parkinson's disease that lasts for a long time;
- postural instability;
- problems with muscle stiffness.
Hypertrophy of the left ventricle of the heart - prognosis
If moderate and minor hypertrophy of the left ventricle of the heart is diagnosed, with treatment and regular medical supervision the prognosis is favorable: patients remain able to work, quality of life is not affected, pregnancy and childbirth are not excluded for women. A severe degree of pathology can lead to disability, the mortality rate is 4-5%.
The prognosis of the course of the disease is determined by the root cause of such a disorder. In the initial stages of hypertrophy, which is corrected with antihypertensive drugs, the prognosis is quite good. The chronic form develops very slowly, and a person with such a disease can live for several decades. At the same time, his quality of life does not suffer.
In elderly people with myocardial ischemia, as well as previous heart attacks, the development of the chronic stage is quite difficult to predict. It can develop both slowly and rapidly, which leads to disability and loss of working capacity of a person.
Operation technique
Due to the relatively good prognosis for asymptomatic left ventricular aneurysm, the indications for surgery are relative. However, in patients undergoing CABG it is necessary to restore the geometry of the left ventricle. On the other hand, for symptomatic (arrhythmias, angina, congestive heart failure) patients, surgery offers a better outcome than drug therapy.
Surgical treatment is absolutely indicated for patients with dyskinetic (akinetic) aneurysms with an increase in the end-systolic index {amp}gt; 80 ml/m2 and end-diastolic index {amp}gt; 120 ml/m2), as well as the threat of rupture or development of a false aneurysm, just as with congenital aneurysms of the left ventricle.
According to recent studies, the myocardium is a muscle band wrapped in a spiral at a certain angle. The interventricular septum consists of three muscular layers and makes a tremendous contribution to the ejection and absorption of blood. Diastole, like systole, is ensured by active muscle contraction.
Taking into account the pathological changes described above caused by post-infarction aneurysm, it becomes clear that its repair is pathogenetically justified. Theoretically, this approach will reduce tension in the wall of the left ventricle and reduce myocardial oxygen demand (based on Laplace's law), neutralize volume overload and reorient the direction of muscle fibers in a more physiological direction with increased mechanical efficiency of ejection and suction.
We suggest you read: Arrhythmia in a child: what is it and what to do?
Exclusion of non-functioning segments from contraction and restoration of the correct orientation of myofibrils can increase the efficiency of the basal and middle segments of the intact myocardium. In this regard, an important point of the operation is the formation of the left ventricle in the form of a “Gothic” rather than a “Romanesque” arch.
Relative contraindications to surgery include excessive risk of anesthesia, lack of viable myocardium outside the aneurysm, low ({amp}lt; 2.0 l•min/m2) cardiac index, severe mitral regurgitation. The global ejection fraction may be less useful in assessing the indication for surgery than the segmental ejection fraction of the remaining left ventricular myocardium.
Angioplasty is only possible in patients with suitable coronary anatomy, one- or two-vessel disease, and contraindications to surgery.
A standard median sternotomy is performed. CPB is carried out in the mode of normo- or moderate hypothermia. Ante- and retrograde blood cardioplegia is used. An external examination of the left ventricle is performed to identify the area of scar and wall thinning. The aneurysm incision is made vertically, moving 1 cm to the left from the LAD.
Resection of the aneurysm is performed using one of the methods described below. Then, if necessary, plastic surgery or replacement of the mitral valve and complete revascularization of the myocardium are performed. CPB switch-off often requires inotropic support. Typically, dopamine (5 mcg/kg/min) is used, as well as nitroglycerin to prevent coronary artery spasm and sodium nitroprusside to reduce afterload.
Supplemental inotropic support does not increase cardiac output and may lead to arrhythmias and tachycardia. Since the left ventricle is poorly distensible, the stroke volume is fixed (left ventricle EDV ≈ 150 ml), heart rate 90-115 beats. per minute sufficient to maintain cardiac index {amp}gt; 2.0 l•min/m2. Counterpulsation may also be necessary in patients with reduced myocardial contractility.
Myocardial hypertrophy - classification
For convenience of work in medicine, it is customary to distinguish between the following types of myocardial hypertrophy:
- obstructive – at the top of the septum, over the entire area;
- non-obstructive – symptoms are mild, diagnosed by chance;
- symmetrical – all walls of the left ventricle are affected;
- apical - the heart muscles are enlarged only from above;
- asymmetrical - affects only one wall.
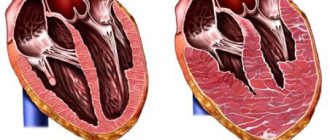
Eccentric hypertrophy
With this type of LVH, the ventricular cavity expands and at the same time a uniform, proportional compaction of the myocardial muscles occurs, caused by the growth of cardiomyocytes. With a general increase in heart mass, the relative thickness of the walls remains unchanged. Eccentric myocardial hypertrophy can affect:
- interventricular septum;
- top;
- side wall.
Concentric hypertrophy
The concentric type of disease is characterized by maintaining the volume of the internal cavity while increasing the mass of the heart due to a uniform increase in wall thickness. There is another name for this phenomenon - symmetrical myocardial hypertrophy. The disease occurs as a result of hyperplasia of myocardiocyte organelles, provoked by high blood pressure. This development of events is typical for arterial hypertension.
Causes of movement disorders
Motor activity disorders are caused by a dysfunction of the brain structures responsible for the dynamics of movement.
Thus, the onset of akinesia may be a consequence of damage to the base of the brain or its frontal lobes due to encephalopathies associated with:
- severe types of renal (uremic catatonic syndrome) or liver failure (hepatic depressive akinesia);
- pathology of hematopoiesis (severe variations in the course of pernicious and hemolytic anemia);
- diseases of the respiratory system (encephalopathy due to hypoxic disorders).
Or it can be caused by severe intoxications and neuroinfections.
The cause of hypokinesia may be dysfunction of the structures that make up the extrapyramidal (stroiopallidal) system, which includes such formations of the subcortex as the pallidum and the red nuclei - the beginning of the Monaco extrapyramidal tract, which carries out the implementation of unconditioned reflexes, conducting impulses from all parts of the cerebrum below the cortex to the anterior horns of the spinal cord .
In turn, pathological changes in the extrapyramidal system can be provoked by various mental and neurological conditions.
Hypokinesia can also be a consequence of physical inactivity - a lack of movement or a deficiency of local muscular activity with the production of monotonous movements that do not require high energy costs.
Chronic industrial and household intoxications and other factors lead to the same results.
The most characteristic symptoms are hypokinesia in parkinsonism.
Possible causes of akinesia include:
- Anomalies of the development of the nervous system;
- Connective tissue diseases such as chondrodysplasia;
- Hydrops fetalis;
- Maternal illness or drug use.
Any changes in the womb that cause insufficient blood flow and oxygen levels can lead to fetal akinesia.
Doctors have identified two gene mutations that are associated with increased risks for the development of fetal akinesia. If a person has relatives with the pathology, then he should test for mutations in the DOK7 and RAPSN genes, which are associated with akinesia.
Signs of the disease
Cardiac pathology does not manifest itself for a long time. But over time, increased muscle mass begins to have an effect on systemic blood flow. The first signs of illness appear. They are usually associated with heavy physical activity. As the disease progresses, manifestations bother the patient even at rest.
Symptoms of left ventricular hypertrophy:
- Shortness of breath, heart palpitations, lack of air.
- Dizziness, fainting.
- Anginal (squeezing, pressing) pain behind the sternum.
- Changes in blood pressure.
- High blood pressure, difficult to respond to therapeutic measures.
- Swelling of the limbs and face in the evening.
- Attacks of suffocation, causeless coughing when lying down.
- Blueness of nails, nasolabial triangle.
- Drowsiness, headaches of unknown origin, weakness.
If you notice such signs in yourself, you should rush to see a cardiologist.
Treatment
Treatment depends on what is causing the symptoms of akinesia. For example, if you have drug-related akinesia, you need to stop taking the medications that are causing the problem.
For Parkinson's disease, doctors often prescribe medications that increase the amount of dopamine in the body. They may help because decreased dopamine levels cause the neuromuscular symptoms associated with Parkinson's disease. Examples of such drugs include levodopa and carbidopa, as well as dopamine agonists.
People with Parkinson's disease or other disorders can see an exercise therapist to help them learn to move safely. There is currently no treatment for this condition.
Depending on the cause of the disorder, treatment will differ fundamentally in the methods used.
If there is a brain tumor, it is surgically excised; if there is an intoxication factor (domestic, industrial), it is necessary to take measures to suppress the effects of the toxic compound on the body.
Neuroinfections are treated with the use of medications that are effective against the pathogen that causes the pathology, and the consequences of their effects are eliminated by using the entire arsenal of drugs that improve blood supply to the brain and detoxifying medications.
Preventive measures include measures aimed at preventing pathogens of neuroinfections from entering the body, preventing head injuries and the risk of toxic effects of industrial and household poisons.
Increasing physical activity by knowledge workers (preventing physical inactivity) is also an essential measure to prevent movement dynamics disorders.
Pathogenesis of the disease
Hypertrophy is almost always a concomitant syndrome of various long-term heart diseases. The main causes of left ventricular hypertrophy are:
Pathophysiology of the main syndromes: heart failure
The authors do not declare any conflict of interest. All authors contributed to the writing of this manuscript and read and approved the final version of the manuscript. Heart failure is a reduction in the performance of the myocardium, which is no longer able to satisfy the body's needs, stress and even rest. Many pathological situations can lead to heart failure. If their pathophysiology remains, more often than not, confusing, we can highlight the underlying mechanisms involved.
- smoking and excessive alcohol consumption;
- overweight;
- long grueling physical activities;
- aortic valve stenosis;
- hypertensive cardiopathy.
Most often, hypertrophy occurs against the background of arterial hypertension. In 60-70% of clinical cases, changes in myocardial mass are caused by persistent blood pressure.
Increased after-discharge can lead to heart failure when it is prolonged and prolonged. This applies to chronic hypertension, as well as narrowing of the aortic valve. Increasing preload can produce a similar result when, for example, aortic valve incontinence causes systolic regurgitation such that ventricular telediastolic volume increases.
Finally, functional deterioration of the heart muscle itself causes heart failure with myocardial dysfunction. In fact, this classification remains partially arbitrary since myocardial dysfunction leads to increased systolic volume and hence volume overload when the ventricle does not drain properly during systole. This is the same when, after charging, it increases when the ventricular failure cannot effectively fight. However, the chronological sequence of changes differs depending on the underlying pathology, and the morphological rearrangements arising from them are also different.
Excess weight is also a prerequisite for the occurrence of LVH, since an increased body size needs an increased blood supply, which leads to the development of pathological changes in the myocardium.
Heart disease is the main congenital predisposition to the development of myocardial hypertrophy due to impaired blood outflow from the ventricle.
These changes, referred to under the general term remodeling, appear rather in the direction of ventricular dilatation when the underlying cause is volume overload. Dilatation is global or local. The remodeling is mainly due to hypertrophy of the ventricular muscle in the case of increased post-treatment.
Thus, parietal tension is a central parameter of heart failure. Myocardial energy expenditure is indeed proportional to wall tension. The greater the pressure, the larger the diameter of the ventricular cavity and the smaller the walls of the ventricle.
Return to contents
results
Hospital mortality has decreased significantly in recent years and is 3-7%. The main cause of death is left ventricular failure – 64%. Risk factors for postoperative mortality are: older age, incomplete myocardial revascularization, female gender, emergency surgery, mitral valve replacement, left ventricular ejection fraction {amp}lt; 30%, preoperative cardiac index {amp}lt; 2.1 l.min/m2, mean pulmonary artery pressure {amp}gt; 33 mmHg, creatinine level {amp}gt; 180 mg/l.
The most common complication during the hospital period is low cardiac output syndrome, as well as ventricular arrhythmias and pulmonary failure.
In the long-term postoperative period, the function of the left ventricle improves: the ejection fraction increases, the EDV and ESV of the left ventricle, diastolic filling and compliance decrease, and exercise tolerance increases. The average angina class decreases from 3.5 to 1.2, and the NYHA heart failure class decreases from 3.0 to 1.7. The results directly depend on the method of left ventricular remodeling, demonstrating a clear lack of advantage of linear repair.
5-year survival rate is 58-80%, 10-year survival rate is 34-57%. 57% of deaths are the result of repeated myocardial infarctions. Preoperative risk factors for long-term mortality are age, ejection fraction {amp}lt; 35%, Left Ventricular EDD {amp}gt; 20 mm Hg, and mitral insufficiency.
Treatment of left ventricular aneurysm in Minsk – European quality at a reasonable price.
Professor, Doctor of Medical Sciences Yu.P. Ostrovsky
Tags: akinesia, cardiology, such
About the author: admin4ik
« Previous entry
Folk remedies
Folk remedies can be used as additional therapy to the main treatment. They can be used only after diagnosis and with the permission of a doctor. Traditional treatment involves the use of medicinal herbs that help get rid of the cause of the disease, its symptoms and are aimed at normalizing well-being.
To prepare the decoction you will need a mixture of wild rosemary, motherwort, dried herbs and kidney tea. All components must be mixed in equal proportions, then take 2 tbsp. l. mixture and pour 300 ml of boiling water. Boil over low heat, cool, strain and take 3 times a day.
A strong infusion of St. John's wort will help strengthen the heart muscle. You need to add a little honey before consuming it. Strawberry jam with milk, dried fruits, dried apricots, cranberries with sugar, and raisins have a good effect.