Myocardial contractility is the ability of the heart muscle to provide rhythmic contractions of the heart automatically in order to move blood through the cardiovascular system.
The heart muscle itself has a specific structure that differs from other muscles of the body. The elementary contractile unit of the myocardium is the sarcomere, which consists of muscle cells - cardiomyocytes. The change in sarcomere length under the influence of electrical impulses of the conduction system ensures cardiac contractility.
Impaired myocardial contractility can lead to unpleasant consequences such as heart failure and more. Therefore, if symptoms of contractility disorders occur, you should consult a doctor.
Features of the myocardium
The myocardium has a number of physical and physiological properties that allow it to ensure the full functioning of the cardiovascular system. These features of the heart muscle make it possible not only to maintain blood circulation, ensuring a continuous flow of blood from the ventricles into the lumen of the aorta and pulmonary trunk, but also to carry out compensatory and adaptive reactions, ensuring the body’s adaptation to increased stress.
The physiological properties of the myocardium are determined by its extensibility and elasticity.
The extensibility of the heart muscle ensures its ability to significantly increase its own length without damaging or disrupting its structure. For reference. The strength of further myocardial contractions during systole (contraction of the heart muscle, ending with the expulsion of blood from the cavities of the ventricles) depends on the degree of myocardial extensibility during diastole (relaxation of the heart muscle).
The elastic properties of the myocardium ensure its ability to return to its original shape and position after the effect of deforming forces (contraction, relaxation) ends.
Also, the ability of the heart muscle to develop strength during myocardial contraction and perform work during systole plays an important role in maintaining adequate cardiac activity.
For reference. Physiological characteristics are manifested by excitability, contractility of the myocardium, its conductivity and automaticity.
What is myocardial contractility?
Cardiac contractility is one of the physiological properties of the heart muscle, which realizes the pumping function of the heart due to the ability of the myocardium to contract during systole (leading to the expulsion of blood from the ventricles into the aorta and pulmonary trunk (TP)) and relax during diastole.
Important. Myocardial contractility is distinguished by a clear sequence that maintains the rhythm and continuity of heart contractions.
First, the contraction of the atrial muscles occurs, and then the papillary muscles and the subendocardial layer of the ventricular muscles. Further, the contraction spreads to the entire inner layer of the ventricular muscles. This ensures a full systole and allows you to maintain a continuous ejection of blood from the ventricles into the aorta and LS.
The contractility of the myocardium is also supported by:
- excitability, the ability to generate an action potential (get excited) in response to stimuli;
- conductivity, that is, the ability to conduct the generated action potential.
Cardiac contractility also depends on the automatism of the heart muscle, manifested by the independent generation of action potentials (excitations). Thanks to this feature of the myocardium, even a denervated heart is able to contract for some time.
What determines the contractility of the heart muscle?
Attention. Myocardial contractility (MC) can be influenced by the nervous system, various hormones and drugs.
The physiological characteristics of the heart muscle are regulated by the vagus and sympathetic nerves, which can influence the myocardium:
- chronotropic;
- inotropic;
- bathmotropic;
- dromotropic;
- tonotropically.
Text of the book “Fundamentals of Cardiac Physiology”
1.6. Coupling of excitation and contraction in the myocardium
The fundamental properties of the myocardium (excitability, conductivity and automaticity) ensure its contractility - the ability of muscle fibers to shorten or increase their tension.
In accordance with the theory of “sliding filaments”, proposed by H. Huxley and A. Huxley back in the 1950s, when myofibrils contract, the sarcomere shortens, that is, a decrease in its longitudinal size due to the active movement of actin filaments relative to myosin filaments. In this case, the length of the threads does not change. Molecular research in the 1970s–1980s. It has been established that actin filaments slide along myosin filaments due to the “raking” movements of the myosin heads. The head attaches to a binding site on actin, then tilts, causing the sarcomere to shorten, and detaches from actin. The head then attaches to the next binding site on the actin filament, and the cycle repeats. In this case, the force of contraction is determined by the number of bonds (bridges) between myosin and actin. In relaxed myocardium, the connection of myosin and actin is prevented by troponin molecules, which “close” the binding sites on the actin filament. However, when the concentration of calcium in the cytoplasm increases, which occurs during excitation of the cardiomyocyte, calcium ions combine with troponin C. The addition of Ca2+ to this protein leads to conformational changes in the troponin-tropomyosin complex. As a result, tropomyosin molecules are displaced, myosin and actin filaments interact, and the contraction process begins. The more Ca2+ ions enter the myofibrils during excitation, the greater the number of actomyosin bridges will be formed, and the stronger, therefore, the contraction will be. Thus, an increase in the concentration of Ca2+ ions in the cytoplasm of the cardiomyocyte is a key factor ensuring electromechanical coupling - the connection between excitation and contraction of the myocardium.
Studies conducted in the 1980–1990s revealed that on the membrane of the T-tubules of the surface membrane of cardiomyocytes there is a voltage-dependent calcium channel, which is blocked by drugs from the dihydropyridine group. Therefore, it is called the dihydropyridine receptor (DHPR). On the membrane of the terminal cisterns of the sarcoplasmic reticulum there is another voltage-dependent calcium channel, the permeability of which is modulated by the plant alkaloid ryanodine, so it is called the ryanodine receptor (RyaR). In addition, the calmodulin protein may be associated with the latter, conformational changes of which can lead to activation of the ryanodine receptor and the release of calcium ions from the cisterns of the sarcoplasmic reticulum. According to other data, ryanodine receptors are directly activated by calcium ions (Fig. 7).
Rice. 7.
Transport of calcium ions in the processes of coupling excitation and contraction in the heart muscle
Electromechanical coupling in the cardiomyocyte begins with the occurrence of phase 0 action potential on the plasma membrane. When the membrane potential reaches a level of –65 mV, voltage-gated L-type Ca2+ channels open, ensuring the formation of an incoming ICa2+L current, which accelerates the depolarization of cardiomyocytes. As a result, voltage-dependent calcium ion channels of the T-tubule membrane (dihydropyridine receptor) are activated, through which calcium ions enter the cardiomyocytes. “External” calcium ions interact (directly or through calmodulin) with ryanodine receptors of the sarcoplasmic reticulum. As a result, calcium ion channels of the sarcoplasmic reticulum open, and calcium begins to flow from the membrane cisterns into the cytoplasm of the cardiomyocyte. As a result, the calcium concentration in the cell cytoplasm increases from less than 10-7 M/l to 10-5 M/l. A sharp increase in the concentration of Ca2+ ions in the sarcoplasm eliminates the tropomyosin blockade of the interaction between actin and myosin and starts the process of contraction of cardiomyocytes.
Thus, the entry of “external” or trigger calcium ions causes the release of “internal” calcium ions from the sarcoplasmic reticulum. This process is called calcium-induced calcium release. It is important to emphasize that the more pronounced the flow of external calcium ions into the cytoplasm of the cardiomyocyte, the more the number of calcium ions leaving the sarcoplasmic reticulum will increase. Since the incoming calcium current ICa2+L reaches its maximum value during phase 2 (plateau) of the action potential of the working cardiomyocyte, the duration of this phase normally determines the force of myocardial contraction. Consequently, the contractility of the heart muscle directly depends on the strength of the incoming calcium current (ICa2+L), which can increase, for example, under the influence of catecholamines, which affect the degree of opening of L-type calcium channels. Along with this, the entry of external calcium ions into the cytoplasm replenishes calcium reserves in the cisterns of the sarcoplasmic reticulum, which ultimately also affects myocardial contractility.
There is another mechanism for the entry of large quantities of Ca2+ ions into the cytoplasm of the working cardiomyocyte during its excitation. It is provided by the coupled transport of calcium and sodium ions across the membrane, that is, Ca2+/Na+ exchange. During diastole, the Ca2+/Na+ pump actively removes Ca2+ ions from the cell in exchange for Na+ ions. When the cardiomyocyte is excited, the direction of Ca2+/Na+ exchange changes to the opposite: Ca2+ ions are actively transferred into the cell, while Na+ ions, on the contrary, are removed, and as a result, the concentration of calcium ions in the cytoplasm of the cardiomyocyte increases.
Violation of the process of electromechanical coupling in cardiac pathology can lead to the fact that action potentials, continuing to arise in the sinus node and propagate through the conduction system to the working myocardium, do not cause its contraction. The lack of contractile function of the myocardium leads to circulatory arrest. However, the electrical activity of the heart can be detected, for example, by recording an electrocardiogram. This condition is called electromechanical dissociation and can be one of the immediate causes of death, for example, in myocardial infarction.
A decrease in myocardial contractility is one of the main causes of the development of heart failure - a condition in which the hemodynamic function of the heart and the normal blood supply to organs and tissues are disrupted. In clinical practice, cardiac glycosides are used to treat heart failure - substances isolated from plants such as digitalis (digitalis), strophanthus, lily of the valley, etc. (Foxglove preparations were first introduced into clinical practice by the English physician W. Withering back in 1785) As physiological and pharmacological studies conducted in the mid-1970s–1980s showed, the mechanism of action of these drugs is due to their ability to influence the functioning of the K+/Na+ pump of cardiomyocyte membranes, as well as myocardial metabolism. In small therapeutic doses, cardiac glycosides enhance the work of the K+/Na+ pump, which partly increases the concentration of potassium ions in the cells, causing an increase in its contractility.
In medium and high therapeutic doses, these drugs, on the contrary, inhibit the K+/Na+ pump of the cardiomyocyte membrane, which leads to an increase in the intracellular Na+ concentration and an increase in the flow of Ca2+ ions into the cell through the Ca2+/Na+ exchange mechanism (both at rest and during excitement). As a result, the duration of the plateau phase of the action potential of the working cardiomyocyte increases, and consequently, myocardial contractility increases even more.
1.7. Features of contractility and biomechanics of the heart muscle
The work of the heart as a pump is ensured primarily by the normal contractile function of the myocardium. In studies conducted in the 1970s and 1980s. on the papillary (papillary) muscle of the mammalian myocardium, attempts were made, firstly, to create biophysical models to describe the parameters of myocardial contractile activity, such as the force and speed of contraction, and secondly, to identify the relationship between these parameters and indicators of the pumping function of the heart, such as ventricular stroke volume and cardiac output. These models were initially based on the theory of skeletal muscle contraction proposed by the English physiologist and Nobel Prize winner A. Hill back in 1922. However, as it turned out, cardiac muscle differs from skeletal muscle in a number of fundamental characteristics of contractility.
The "all or nothing" law.
Since the myocardium is a functional syncytium, when an action potential develops in one cardiomyocyte, the excitation process spreads at a high speed (up to 0.5 m/s) to neighboring unexcited cells. Thus, there is a rapid coverage of all working cardiomyocytes by excitation, which ensures synchronicity and almost simultaneity of their contraction. As a result, the force of heart contraction does not depend on the strength of the superthreshold stimulus (the “all or nothing” law). This law was first formulated by the American physiologist H. Bowditch in experiments with electrical stimulation of an isolated heart at the end of the 19th century.
Inability to sum up contractions (tetanus).
As stated above, the duration of the refractory period (absolute and relative) of the working myocardium approximately corresponds to the time of the entire action potential (300 ms). It is fundamentally important that the duration of the action potential of working cardiomyocytes practically coincides in time with the duration of their contraction. Therefore, a subsequent impulse can cause myocardial contraction only after its relaxation, which corresponds to the end of the previous action potential. As a result, the summation of contractions in the myocardium with increasing frequency of stimulation is impossible, that is, the development of tetanus, as in skeletal muscle, which could lead to impaired contraction and cardiac arrest. (Recall that the duration of the action potential of skeletal muscle is about 5-10 ms, and the duration of its contraction is 40-50 ms.) In skeletal muscle, the next impulse already 10 ms after the first can cause a new contraction when the muscle has not yet relaxed, which leads to the summation of contractions. This does not happen in the myocardium due to the significant duration of the refractory period.
Dependence of the strength of contractions on the magnitude of the incoming calcium current.
It was said above that myocardial contraction occurs in response to the intake of “external” calcium ions, which cause the release of “internal” calcium from the sarcoplasmic reticulum. Therefore, the more pronounced the incoming ICa2+L current is, the greater the amount of calcium ions will exit into the cytoplasm through the ryanodine calcium channel receptor from the sarcoplasmic reticulum, and the greater the number of actomyosin bridges will be formed. Thus, it is the magnitude of the incoming calcium current ICa2+L that determines the force of contraction of working cardiomyocytes and the myocardium as a whole. Since the incoming calcium current ICa2+L normally reaches its maximum value during phase 2 of the action potential of the working cardiomyocyte, the duration of this phase determines the force of myocardial contraction. The duration of phase 2 may increase under the influence of β-adrenergic receptor agonists, catecholamines released from the sympathetic nerves of the heart or circulating in the blood. Therefore, excitation of such receptors is accompanied by increased myocardial contractility, which plays an important role in the nervous and humoral regulation of cardiac activity.
Frequency-force relationship.
As already noted, even at very high frequencies of stimulation, the myocardium is not capable of developing tetanus (summed contraction), characteristic of skeletal muscle.
This feature is a consequence of the long refractory period of cardiomyocytes, which coincides in time with the duration of contraction, and protects the heart from premature excitation and fatigue. However, back in the 19th century. American physiologist X. Bowditch, in experiments with electrical stimulation of an isolated heart, observed an increase in the force of heart contractions with increasing stimulation frequency. This “frequency-force” relationship is called the “Bowditch ladder”, or chronoinotropic effect (Greek chronos
- time,
inos
- force). The occurrence of a chronoinotropic effect may be due to the fact that at a high frequency of stimulation, the time intervals between contractions are shortened, as a result of which the Ca2+ ions entering the sarcoplasm during the next contraction are not completely removed. As a result, with each subsequent contraction, the concentration of intracellular ionized Ca2+ increases, and the strength of contractions increases accordingly. The chronoinotropic effect can be considered a type of homeometric regulation of the heart, and it will be discussed further along with other myogenic mechanisms.
Length-force relationship.
Studies on the papillary muscle of the cat myocardium have shown that when the sarcomere is stretched, actin and myosin filaments move out of the spaces between them. As a result, the number of actin-myosin bridges that can be formed during contraction increases, and, consequently, conditions are created for an increase in the force of contraction with greater stretching of the myofibrils. The maximum contractile force is achieved with an initial sarcomere length of about 2.2 µm. At the same time, when myocardial fibers are stretched, there is also an increase in the incoming calcium current in response to the activation of the so-called stretch calcium channels.
stretch-activated channels
), which were found not only in smooth muscles, but also in the myocardium.
An increase in incoming calcium current directly causes an increase in myocardial contractility. In addition, in response to changes in the initial length of myocardial fibers, the sensitivity of troponin C to calcium ions increases, which promotes the activation of a larger number of actomyosin bridges.
Thus, the initial length of myocardial fibers is a key determinant in the regulation of the force of its contraction. In an intact heart, the initial stretch of myocardial fibers can be indicated by indicators such as end-diastolic pressure and ventricular volume. The dependence of the force of contraction on the degree of preliminary stretching of the myocardium was noted by the German physiologist O. Frank on the heart of a frog in 1895 and studied in detail on a cardiopulmonary preparation of a dog by English physiologists S. Patterson and E. Starling in 1914. The meaning of Frank’s “law of the heart” – Starling for the regulation of its pumping function (heterometric regulation) will be discussed in detail in connection with the myogenic regulation of cardiac activity.
Dependence "speed - force".
Research conducted by A. Hill on skeletal muscle made it possible to establish a graphical hyperbolic relationship between the load and the speed of muscle contraction, which is expressed by the Hill equation:
where V
– contraction speed, cm/s;
P
– force of muscle contraction (load), gf;
Р0 – maximum possible contraction force; a
is a constant that characterizes the heat generated during muscle shortening and depends on the efficiency of the muscle;
b
is a constant characterizing the rate of transition of chemical energy into mechanical energy (constants
a
and
b
have the dimensions of load and speed, respectively).
From this equation it follows that if the load on the muscle is zero ( P
= 0), then the speed of its contraction is maximum and is equal to
V
max =
bР
0/
a
.
The mode of muscle contraction with constant force (under constant load) is called isotonic (Greek isos
- equal,
tonos
- tension).
If the load on the muscle is maximum ( P
=
P
0), then there is no shortening, that is,
V
= 0, which corresponds to the state of maximum isometric contraction (Greek
isos
- equal,
metron
- measure, size of contraction (tension)).
However, studies performed on papillary muscle have shown that in the myocardium there is a deviation of the hyperbolic force-velocity relationship established for skeletal muscle. This is due to many reasons. Firstly, even with a constant volume of the chambers of the heart, when the myocardium contracts, there is an internal shortening of the central and simultaneous stretching of the peripheral parts of the heart muscle. Consequently, the heart lacks classical isometric contraction, in which the length of muscle fibers remains constant. Secondly, the myocardium as a functional syncytium has structural heterogeneity, and therefore some sarcomeres can be stretched to a greater or lesser extent than others. Thirdly, the nature of the force-velocity relationship in the myocardium, to a greater extent than in skeletal muscle, is influenced by active relaxation processes (for more details, see subsection 1.10). Finally, changes in the hyperbolic force-velocity relationship are caused by many substances that act on the heart, such as adrenaline, calcium ions, and digitalis preparations.
1.8. Cardiac cycle and its phase structure
The activity of the heart as a pump is a continuous alternation of periods of contraction (systole) and relaxation (diastole) of the atria and ventricles throughout a person’s life. The alternating systole and diastole make up the cardiac cycle. At rest, the heart rate (HR) in an adult is 60–80 cycles per minute, that is, each cycle lasts about 0.8 seconds. Of this time, atrial systole lasts for about 0.1 s, ventricular systole lasts for about 0.3 s, and the rest of the time (about 0.4 s) is total diastole, or cardiac pause.
For the first time, a detailed phase analysis of cardiac activity was carried out by the American physiologist K. Wiggers in the first third of the 20th century. He obtained simultaneous recordings of curves of changes in blood pressure in the aorta, left ventricle and atrium, as well as left ventricular volume. It is convenient to view the cardiac cycle on a pressure-volume diagram, which is obtained by simultaneously recording pressure and volume in the cavity of the left ventricle and comparing them on one graph (Fig. 8).
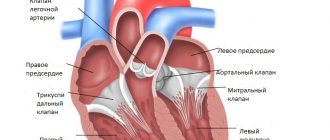
During a general pause, the myocardium is relaxed, and the cardiac chambers are filled with blood coming from the main veins. At this time, the atrioventricular valves are open, and blood flows freely from the atria into the ventricles. On the contrary, the semilunar valves of the aorta and pulmonary trunk are closed, since the diastolic pressure in these vessels is much higher than in the ventricles (the pressure in the ventricles during their diastole is close to zero) (section A - B).
The generation of the next impulse in the sinoatrial node causes electrical excitation of the atria, which leads to their contraction. There are no valves between the main veins and the atria, therefore, to prevent the outflow of blood from the atria back into the veins during atrial systole, the circular muscles surrounding the mouths of the vena cava and pulmonary veins contract. During atrial systole, the blood pressure in them increases and becomes greater than in the ventricles, which are still relaxed at this time (Fig. 9).
Due to the pressure difference, an additional portion of blood passes from the atria to the ventricles, the volume of which does not exceed 15% of the total filling of the ventricles during diastole. The movement of blood in this case is turbulent due to reflection from the walls of the ventricles. This pattern of blood flow facilitates the closure of the atrioventricular valves at the beginning of ventricular systole. With the end of atrial systole, ventricular diastole also ends.
Rice. 8.
Changes in pressure and blood volume in the ventricles throughout the cardiac cycle:
A
– in “pressure – time” coordinates;
b
– in “pressure – volume” coordinates (PV diagram of the left ventricle) A – B – period of tension; B – C – period of exile; C – D – relaxation period; D – A – filling period. Time points: A – closure, C – opening of the left atrioventricular valve; B – opening, D – closing of the aortic valve. ADD – diastolic blood pressure, ADS – systolic pressure in the aorta; ESP – end-systolic pressure, EDP – end-diastolic pressure in the left ventricle; ESV – end-systolic volume, EDV – end-diastolic volume of the ventricle; SV – stroke volume of the heart
Rice. 9.
Pressure in the cardiac cavities in different phases of the cardiac cycle:
A
– right half of the heart;
b
– left half; the upper numbers are the pressure in the atria, the lower numbers are the pressure in the ventricles
At this point, there is a certain amount of blood in the ventricles, which forms the end-diastolic volume and creates a non-zero end-diastolic pressure that determines the preload of the heart (volume load).
From the atria, excitation after atrioventricular delay spreads at high speed along the conduction system of the ventricles, reaching working cardiomyocytes.
The first period of ventricular systole begins - a period of tension. The initial phase of this period—the phase of asynchronous contraction—corresponds to the sequential “switching on” of contractile cardiomyocytes. Intraventricular pressure during this phase of systole increases slightly. From the moment the entire ventricular myocardium is covered by excitation, the phase of isovolumic contraction begins, the mode of which is close to isometric. However, as already noted, classical isometric contraction, in which the length of muscle fibers remains constant, is not observed in the intact heart. Even with a constant volume of the chambers of the heart, internal shortening of the central and simultaneous stretching of the peripheral sections of the heart muscle occurs. In addition, when the heart contracts, its walls undergo deformation, which leads to a change in the length of muscle fibers. Therefore, the term “isovolumic contraction” in relation to this phase is more correct and accurate (Fig. 10).
It is characterized by synchronous contraction of all cardiomyocytes under conditions when the atrioventricular valves are already closed and the semilunar valves have not yet opened, since the pressure in the aorta and pulmonary trunk at this moment is greater than in the ventricles. Thus, the ventricles are isolated on one side from the atria, and on the other from the vessels. In this case, the volume of the ventricles remains constant. The phase of isovolumic contraction is the most important in the activity of the heart, since it is during this period that the contracting myocardium imparts potential energy to the blood. Intraventricular pressure in the phase of isovolumic contraction increases at a maximum speed of up to 2000 mm Hg. Art./s, and when it becomes higher than the diastolic pressure in the aorta and pulmonary trunk, the semilunar valves open, and the period of expulsion of blood from the ventricles into the main arteries begins.
Rice. 10.
Changes in the shape of the heart when its parts contract:
A
– section in the transverse plane.
The dotted line shows the contours of the ventricles and orifices; b
– section in the frontal plane; 1 – semilunar valves of the aorta; 2 – tricuspid valve; 3 – bicuspid valve; 4 – semilunar valves of the pulmonary artery; 5 – atrial systole; 6 – ventricular systole
When blood is expelled, the potential energy imparted to it by the myocardium transforms into kinetic energy. Initially, the blood in the aorta and pulmonary trunk moves at high speed (fast expulsion phase), then the speed of blood movement decreases (slow expulsion phase). This happens because blood from the heart enters the already blood-filled aorta and pulmonary trunk; when blood is expelled from the heart, it stretches the walls of these vessels (for example, the diameter of the aorta increases by 25%). In addition, as blood is expelled, the rate of myocardial contraction decreases. In the rapid ejection phase, the ventricles contract in a mode close to isotonic (with constant force), the blood pressure in them increases slightly compared to the period of isovolumic contraction, while their volume quickly decreases. As the blood supply to the aorta and pulmonary arteries increases, the pressure in these vessels increases, reaching a maximum value at the end of systole, which is called systolic pressure. The rate of blood flow from the heart then decreases, so the final phase of the ejection period, as noted earlier, is called the slow ejection phase. Sometimes the terms maximum and reduced expulsion phases are used to designate the phases of fast and slow expulsion, respectively.
By the end of ventricular systole, a certain amount of blood remains in them (end-systolic, or residual, volume), which corresponds to a certain blood pressure (end-systolic pressure). After the end of ventricular contraction, a period of relaxation begins. At the same time, the pressure in them, as well as in the aorta and pulmonary trunk, begins to decrease, and in the main arteries, due to their elastic properties, as well as the hydraulic resistance of the vessels, this occurs more slowly than in the ventricles. As soon as the blood pressure in the ventricles becomes less than the pressure in the aorta and pulmonary trunk, the semilunar valves close. The time from the beginning of the relaxation period to the closing of the semilunar valves is called the protodiastolic period (interval).
From the moment the semilunar valves close, the ventricles, continuing to relax, again become isolated from the aorta and pulmonary trunk, as well as from the atria, since the atrioventricular valves are still closed during this period. This is due to the fact that the pressure in the relaxing ventricles is still higher than in the atria. This period of diastole is called the phase of isometric, or isovolumic, relaxation. When the pressure in the ventricles decreases so much that it becomes less than in the atria, the atrioventricular valves open and the period of filling the ventricles begins, during which blood enters them from the atria. At the same time, the pressure, both in the atria and in the ventricles, continues to decrease. Initially, the blood moves quickly (rapid filling phase). It is at this time that the main blood filling of the ventricles occurs (about 85%). Then, as the ventricles fill, the pressure in them increases and the movement of blood slows down (slow filling phase). The final phase of the ventricular filling period is limited by the onset of atrial systole.
The right and left sections of a healthy heart contract and relax almost synchronously, that is, the systole of the right and left atria, as well as the right and left ventricles, begins simultaneously. When accurately measuring the time characteristics of the phases of the cardiac cycle in experimental conditions on animals and in the clinic in humans, some asynchronism can be observed in the work of the right and left parts of a healthy heart. Thus, the systole of the right atrium begins somewhat earlier and lasts longer than the systole of the left atrium. The systole of both ventricles begins simultaneously, but in the right ventricle it is longer than in the left (due to an increase in the duration of the asynchronous contraction phase), while the relaxation period, on the contrary, is longer in the left ventricle. Normally, these differences in the duration of the phases of different parts of the heart do not exceed hundredths of a second, but they can increase noticeably, for example, if myocardial conduction is impaired.
The time relationships between the described phases are given in Table. 3 and in Fig. eleven.
Rice. eleven.
Scheme of two consecutive cardiac cycles lasting 0.8 s. The periods of systole of the atria and ventricles are indicated in black; shaded areas correspond to the closure of the atrioventricular and semilunar valves
Table 3
Approximate duration (s) of the main phases of the cardiac cycle at a heart rate of 75 min–1
How is myocardial contractility regulated?
The influence of the vagus nerves causes a decrease in:
- myocardial contractility,
- heart rate,
- generation of action potential and its propagation,
- metabolic processes in the myocardium.
That is, it has exclusively negative inotropic, tonotropic, etc. effects.
The influence of sympathetic nerves is manifested by an increase in myocardial contractility, an increase in heart rate, acceleration of metabolic processes, as well as an increase in the excitability and conductivity of the heart muscle (positive effects).
Very important! It should be noted that myocardial contractility also largely depends on blood pressure.
With reduced blood pressure, the sympathetic effect on the heart muscle is stimulated, myocardial contractility increases and heart rate increases, due to which compensatory normalization of blood pressure occurs.
When pressure increases, a reflex decrease in myocardial contractility and heart rate occurs, allowing blood pressure to be lowered to an adequate level.
Myocardial contractility is also influenced by significant stimulation:
- visual,
- auditory,
- tactile,
- temperature, etc. receptors.
This causes changes in the frequency and strength of heart contractions during physical or emotional stress, being in a hot or cold room, as well as when exposed to any significant irritants.
Of the hormones, adrenaline, thyroxine and aldosterone have the greatest effect on myocardial contractility.
The role of calcium and potassium ions
Also, potassium and calcium ions can change the contractility of the heart. With hyperkalemia (excess potassium ions), there is a decrease in myocardial contractility and heart rate, as well as inhibition of the formation and conduction of the action potential (excitation).
Calcium ions, on the contrary, help to increase myocardial contractility, the frequency of its contractions, and also increase the excitability and conductivity of the heart muscle.
Drugs that affect myocardial contractility
Cardiac glycoside preparations
have a significant effect on myocardial contractility .
This group of drugs can have a negative chronotropic and positive inotropic effect (the main drug of the group, digoxin, in therapeutic doses increases myocardial contractility). Due to these properties, cardiac glycosides are one of the main groups of drugs used in the treatment of heart failure. Also, beta blockers (reduce myocardial contractility, have negative chrontropic and dromotropic effects), Ca channel blockers (have a negative inotropic effect), ACE inhibitors (improve the diastolic function of the heart, helping to increase cardiac output in systole) and etc.
Reasons for changing status
A decrease in the strength of heart contractions can occur as a consequence of ischemic disease, especially after a myocardial infarction. Almost 70% of all cases of circulatory failure are associated with this disease. In addition to ischemia, changes in the condition of the heart lead to:
- congenital defects or due to rheumatism;
- cardiomyopathy with expansion of cavities (dilated);
- hypertonic disease;
- diabetes.
The degree of decrease in inotropic function in such patients depends on the progression of the underlying disease. In addition to the main etiological factors, a decrease in the reserve capacity of the myocardium is facilitated by:
- physical and psychological overload, stress;
- rhythm disturbance;
- thrombosis or thromboembolism;
- pneumonia;
- viral infections;
- anemia;
- chronic alcoholism;
- decreased kidney function;
- excess thyroid hormones;
- long-term use of medications (hormonal, anti-inflammatory, high blood pressure), excessive fluid intake during infusion therapy;
- rapid weight gain;
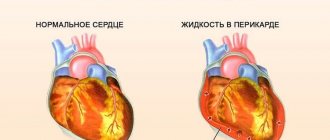
- myocarditis, rheumatism, bacterial endocarditis, fluid accumulation in the pericardial sac.
In such conditions, it is most often possible to almost completely restore the functioning of the heart if the damaging factor is eliminated in time.
Why is contractility disorder dangerous?
Reduced myocardial contractility is accompanied by a decrease in cardiac output and impaired blood supply to organs and tissues. As a result, ischemia develops, metabolic disorders occur in tissues, hemodynamics are disrupted and the risk of thrombosis increases, and heart failure develops.
Attention! Sharply reduced global myocardial contractility is accompanied by pronounced stagnation of blood in the pulmonary circulation, the appearance of severe shortness of breath (even at rest), hemoptysis, edema, and liver enlargement.
When SM may be violated
A decrease in SM can be observed against the background of:
- myocardial hypoxia;
- coronary heart disease;
- severe atherosclerosis of the coronary vessels;
- myocardial infarction and post-infarction cardiosclerosis;
- cardiac aneurysm (a sharp decrease in contractility of the left ventricular myocardium is observed);
- acute myocarditis, pericarditis and endocarditis;
- cardiomyopathies (the maximum impairment of SM is observed with depletion of the adaptive capabilities of the heart and decompensation of cardiomyopathy);
- brain injuries;
- autoimmune diseases;
- strokes;
- intoxication and poisoning;
- shocks (toxic, infectious, painful, cardiogenic, etc.);
- avitaminosis;
- electrolyte imbalances;
- blood loss;
- severe infections;
- intoxication with the active growth of malignant neoplasms;
- anemia of various origins;
- endocrine diseases.
Exercise therapy
Exercise therapy for preload and afterload disorders involves moderate physical activity that does not cause overstrain of the heart muscle.
Walking
Slow walks are the most gentle physical therapy regimen. In the absence of contraindications from a cardiologist, it is recommended to walk 1-2 km daily. Throughout the entire period of walking, you should monitor your general well-being, heart rate, and uniformity of breathing.
A ride on the bicycle
Biking is one of the areas of exercise therapy. Driving this type of transport involves maintaining a moderate pace, so that the training load on the heart muscle is ensured, but at the same time there are no attacks of its rapid contraction. During the day you need to cycle up to 5 km.
Swimming
Swimming involves a serious cardiological load on the heart and blood vessels. Therefore, this type of exercise therapy is carried out together with an instructor or under the supervision of a medical professional.
People with signs of preload and afterload disorders are recommended to visit the pool 2-3 times a week with a swimming duration of 30-40 minutes.
Impaired myocardial contractility - diagnosis
The most informative methods for studying SM are:
- standard electrocardiogram;
- ECG with stress tests;
- Holter monitoring;
- ECHO-K.
Also, to identify the cause of a decrease in SM, a general and biochemical blood test, a coagulogram, a lipid profile are performed, the hormonal profile is assessed, an ultrasound scan of the kidneys, adrenal glands, thyroid gland, etc. is performed.
SM on ECHO-KG
The most important and informative study is ultrasound examination of the heart (assessment of ventricular volume during systole and diastole, myocardial thickness, calculation of minute blood volume and effective cardiac output, assessment of the amplitude of the interventricular septum, etc.).
Assessment of interventricular septal amplitude (IVS) is an important indicator of ventricular volume overload. Normokinesis AMP ranges from 0.5 to 0.8 centimeters. The amplitude of the posterior wall of the left ventricle is from 0.9 to 1.4 centimeters.
A significant increase in amplitude is observed against the background of impaired myocardial contractility, if patients have:
- aortic or mitral valve insufficiency;
- volume overload of the right ventricle in patients with pulmonary hypertension;
- coronary heart disease;
- non-coronarogenic lesions of the heart muscle;
- cardiac aneurysm.
Is it necessary to treat myocardial contractility disorders?
Violations of myocardial contractility are subject to mandatory treatment. In the absence of timely identification of the causes of impaired SM and the prescription of appropriate treatment, the development of severe heart failure, disruption of the functioning of internal organs against the background of ischemia, and the formation of blood clots in vessels with a risk of thrombosis (due to hemodynamic disorders associated with impaired SM) are possible.
If the contractility of the left ventricular myocardium is reduced, then the development is observed:
- cardiac asthma with the appearance in the patient:
- expiratory dyspnea (impaired exhalation),
- obsessive cough (sometimes with pink sputum),
- bubbling breath,
- pallor and cyanosis of the face (sallow complexion is possible).
Attention. Violations of right ventricular SM are accompanied by the appearance of shortness of breath, decreased performance and exercise tolerance, as well as the appearance of edema and enlargement of the liver.
Diagnostics for impaired preload and afterload
The table below presents the main methods of diagnostic examination that are indicated for patients with signs of disturbances in preload and afterload of the heart muscle.
| Diagnostic method | Purpose of the survey |
| ECG | An electrocardiogram displays the rhythmic activity of the heart, as well as the first signs of a violation of the regularity of contractions of this organ. This is a simple but very effective diagnostic method that shows a decrease in preload and afterload of the heart muscle. |
| Echocardiography | Echocardiography is an improved ultrasound method that can be used to detect pathological changes in the structure of heart tissue. For example, damage to its valve apparatus, thickening of the walls of the ventricles and other types of cardiac diseases. |
| Biochemical analysis of venous blood | This type of study is necessary in order to detect possible changes in the biochemical composition of the blood. The collected biological material is checked for the possible presence of infectious microorganisms that have damaged the tissue of the heart muscle, which subsequently leads to a violation of the preload and afterload of the organ. |
Catheterization of the heart and its great vessels in order to determine the blood pressure inside the ventricles of the heart is a radical method for diagnosing preload and afterload disorders, which is used only in specialized cardiology centers.
Treatment of SM disorders
All treatment should be selected by a cardiologist in accordance with the cause of the SM disorder.
To improve metabolic processes in the myocardium, drugs can be used:
- riboxin,
- mildronate,
- L-carnitine,
- phosphocreatine,
- B vitamins,
- vitamins A and E.
Potassium and magnesium preparations (Asparkam, Panangin) can also be used.
Patients with anemia are advised to take iron, folic acid, and vitamin B12 supplements (depending on the type of anemia).
If lipid balance disorders are detected, lipid-lowering therapy may be prescribed. To prevent thrombosis, antiplatelet agents and anticoagulants are prescribed according to indications.
Also, drugs that improve the rheological properties of blood (pentoxifylline) can be used.
Patients with heart failure may be prescribed cardiac glycosides, beta blockers, ACE inhibitors, diuretics, nitrate preparations, etc.
Classification of the disease
According to the duration of development of symptoms of damage, heart failure is divided into acute and chronic. The first of them can develop in one of two types:
- To the left - left ventricular or left atrial.
- To the right - right ventricular.
Cardiovascular failure is classified, according to Vasilenko-Strazhesko, into three stages:
- First. This is the initial stage, characterized by the fact that hidden symptoms become noticeable only if the person is physically active. He experiences shortness of breath and rapid heartbeat. The patient gets tired quickly. In the absence of physical activity, these signs are absent.
- Second. Symptoms of long-term circulatory failure and stagnation of the entire cardiovascular system also appear when a person is at rest. The patient is considered disabled. This stage has two periods. 2nd A is characterized by moderate hemodynamic lesions in one of the sections of the organ. This is the left or right ventricle. Manifestations of shortness of breath are observed even with normal physical activity. The patient's performance decreases. Other manifestations are cyanotic skin color, swelling of the legs, heavy breathing. 2nd B A is characterized by serious violations. The entire cardiovascular system is involved in this process. External manifestations - a person feels shortness of breath even when at rest. Edema and cyanosis appear. The patient is completely disabled.
- Third. This final stage is characterized by a significant deterioration in blood circulation and metabolism. The structure of many organs, in particular the liver and lungs, is irreversibly damaged. The patient is exhausted.
Low pulse: causes and signs
If a patient has acute heart failure, the activity of any part of the heart is weakened. This could be the left atrium, one of the ventricles. Left ventricular failure occurs when there is a disease that puts stress on this department. It could be hypertension, heart attack.
In this case, there is an increase in pressure in some blood vessels and an increase in their permeability. The consequence of this is interstitial edema, which turns into alveolar edema. A similar type of failure manifests itself in the form of cardiac asthma. Alveolar pulmonary edema is also observed.
Exacerbation of asthma is usually caused by excessive physical activity or great emotional stress. Most often, a person experiences severe and sudden suffocation at night, during sleep. As a result, the patient wakes up in horror.
This type of asthma manifests itself as a feeling of lack of oxygen, increased heart rate, wet cough, and severe weakness. During an attack, the patient becomes covered in cold sweat, and when lying down he experiences severe shortness of breath.
The specialist conducting the examination notes that the surface of the skin has turned gray and cyanotic, the person has shortness of breath and cold sweat. The pulse becomes weak and arrhythmic. The borders of the heart expand to the left. Blood pressure decreases. When listening to the lungs, harsh breathing is observed.
There is an increase in stagnation in the pulmonary circulation. As a result, pulmonary edema develops. The patient experiences a state of suffocation, and sputum is released. It is foamy, has a pinkish tint, and is very abundant.
This is due to the presence of blood in it. Even strangers hear heavy, hoarse breathing with bubbling sounds. The patient is forced to be in a vertical position, since in a horizontal position his shortness of breath increases.
The face becomes bluish, the veins in the neck swell, and the patient breaks out in a cold sweat. The pulse is rapid, thread-like, the pressure is low, and wheezing is heard when breathing. If pulmonary edema occurs that requires urgent intervention, intensive care measures must be taken. Otherwise, everything could end very sadly.
Acute heart failure of the left atrium is observed in the case of mitral stenosis. The manifestations of the disease are similar to those found in left ventricular disease.
Damage to the right ventricle is usually a consequence of pulmonary embolism. Congestion appears in the systemic circulation. As a result, the legs begin to swell, the veins of the neck swell, shortness of breath appears, the skin becomes bluish, and there is pressure and pain in the area of the heart. There is a sharp decrease in blood pressure and expansion of the heart to the right.
If a patient has a disease that causes the right ventricle to malfunction, heart failure will appear sooner than if the same happens to the left ventricle. After all, this part of the heart is considered the most powerful. But, if there is a decrease in its functions, the development of heart failure occurs very quickly.
Chronic heart failure is characterized by the fact that at the very beginning its development can be of any type - right and left atrial, right and left ventricular. If there is an aortic defect, arterial hypertension, or some other ailments, congestion is observed in the vessels of the pulmonary circulation, leading to left ventricular failure.
At the same time, vascular changes occur in the lungs. A person experiences shortness of breath, suffocation (usually at night), and bluish skin color. There is an increased heart rate, dry cough, and the patient gets tired very quickly.
If there are problems with the left atrium, severe congestion of the small circle appears. This happens in people who have mitral valve stenosis. The patient experiences shortness of breath, constantly coughs, and begins to spit up blood.
If stagnation in the veins continues for a long time, sclerosis is observed in the vessels and lungs. Blood circulation in the pulmonary circle worsens, as an additional obstacle appears. The load on the right ventricle increases, and it is this circumstance that explains the occurrence of failure.
If the right ventricle is mainly affected, stagnation covers a large circle. A disease of this type can be a consequence of mitral heart defects, pneumosclerosis, and other ailments. The patient begins to complain that he has pain under his right rib and feels heaviness.
He develops swelling, his abdomen becomes enlarged, and shortness of breath occurs with even slight physical activity. Diuresis decreases, the skin becomes cyanotic. Swelling of the veins of the neck and enlargement of the liver are observed.
A lesion that affects only one part of the heart spreads to others after some time. This is how total chronic heart failure occurs, the symptoms and treatment of which should be carried out by a cardiologist.
Low heart rate (bradycardia) does not always occur against the background of serious pathologies. Sometimes this condition can have a purely physiological origin. But often it is a decrease in heart rate that is the first sign of many serious diseases.
- Causes of bradycardia
- Signs
- Low pulse with low blood pressure - what to do
- Reduced heart rate with high blood pressure - what to do
- How to increase your heart rate
- What to do at home
| Low heart rate - what to do at home? |
| Pulse 90: is it normal or not? |
Causes of high heart rate with low blood pressure Normal heart rate in women depending on age Low heart rate with normal blood pressure: how to increase heart rate - a frequent sign of vegetative-vascular dystonia;
- neuroses;
- tumors, brain contusions, meningitis;
- renal colic;
- hypothyroidism;
- smoking.
Causes of bradycardia
The pulse changes under the influence of various factors, including body and air temperature, and emotional state. Heart rate depends on age. For example, the maximum indicators occur in preschool children - more than 100 beats/min.
What indicator is considered low? Bradycardia is diagnosed when the heart rate regularly decreases below 55 beats/min. Pathology often indicates the presence of various diseases. Bradycardia can be extracardial and organic.
When extracardiac bradycardia occurs:
Often the heart rate decreases in people who are fasting and use mono-diets. The number of heart contractions also decreases with strong pressure on the carotid artery. Organic bradycardia develops against the background of cardiosclerosis, heart attack, or taking certain medications.
A very low pulse (less than 40 beats/min) indicates severe intoxication, which occurs with sepsis, viral hepatitis, and typhus. The heart rate is greatly reduced with increased levels of calcium and potassium in the blood, and with poisoning with phosphates, protein breakdown products.
In a child, bradycardia may be physiological in nature - the pulse decreases during sleep and rest, after a long stay in cold water or indoors. In the absence of external stimuli, a decrease in heart rate is most often caused by increased tone of the vagus nerve, which occurs with problems with the heart muscle.
In adolescents, the pulse decreases due to a rapid increase in the size of the heart, against the background of hormonal changes, impaired metabolism, and overwork.
Why is low heart rate dangerous? With bradycardia, the brain and internal organs experience a constant lack of oxygen, which can cause the development of irreversible pathological conditions. A decrease in heart rate below 30 beats/min can lead to fainting. The most dangerous complications are cerebral edema, coma, cardiac arrest.
Signs
A slight decrease in heart rate has virtually no effect on your well-being. When the heart rate drops below 50 beats/min, a person’s condition can deteriorate sharply.
Symptoms of bradycardia:
- headache, severe dizziness begins;
- the person becomes lethargic, weakness appears;
- semi-fainting state, possible loss of consciousness;
- chest pain, shortness of breath;
- arrhythmia.
If a decrease in heart rate is caused by problems with the thyroid gland, then the main symptoms include tremor of the fingers, muscle weakness, and the person suddenly loses weight.
In case of cardiac pathologies, a low pulse is accompanied by increased fatigue, which does not go away even after proper rest, sweating increases, and limbs swell.
Hypotension in combination with a low pulse is a common sign of serious illnesses, sometimes this condition is life-threatening.
Reasons for this condition:
- heart attack - diastolic pressure decreases, severe pain occurs in the chest;
- pulmonary embolism;
- anemia, neuroses, metabolic disorders - often accompanied by fainting and collapse;
- severe blood loss, bleeding;
- Quincke's edema;
- infections, intoxication.
If you have hypotension and low heart rate, you should not rush to take medications. Sometimes it’s enough to lie down, drink green tea or strong natural coffee with sugar. If your health allows, you can walk briskly or do a little exercise.
With a slight decrease in heart rate, a person feels only symptoms of high blood pressure - migraine, ringing in the ears, shortness of breath, nausea. In this case, the skin becomes covered with red spots, and hot flashes occur. If the pulse drops below 50 beats/min, dizziness begins and loss of consciousness is possible.
Why does the heart rate decrease with hypertension?
- cardiac pathologies – endocarditis, heart disease, ischemia, cardiosclerosis;
- vegetative-vascular dystonia;
- various types of tumors;
- endocrine diseases, hormonal fluctuations;
- mental and physical overload.
Sometimes bradycardia is a side effect after taking medications to normalize blood pressure.
[/idea]Important! If high blood pressure is accompanied by bradycardia, then you should not take any medications until doctors arrive - this can only worsen the situation.[/idea]