Minor cardiac anomalies (MAC) are the morphological basis of possible functional disorders that can worsen the prognosis in case of organic heart disease.
Etiology
- Cardiogenesis disorder
- dysplasia of connective tissue structures (based on genetically altered fibrillogenesis of the extracellular matrix)
- disruption of ontogenesis processes
- autonomic dysfunction
Prevalence
- According to population studies - 10.9%
- according to autopsies - 10.6%
- in children with manifestations of connective tissue dysplasia up to 97%
Structural features
in adults:
- single anomalies - 75.6%
- combined - 24.4%
- mitral valve prolapse - 32.1%
- abnormally located chords - 41.8%
- others - 26.1%
in children:
- abnormally located trabeculae - 94%
- Mitral valve prolapse (MVP) - 37% (anterior wall - 50%, posterior wall - 31%, anterior + posterior - 19%)
- MVP + microanomalies of the chords (ectopic attachment of the chords: anterior wall - 31%, posterior wall - 16%, disturbance of the distribution of the chords: anterior wall - 31%, posterior wall - 16%, elongation of the chords: anterior wall - 62%, posterior wall - 25 %)
- PTC - 7% (in combination with PMC)
- elongated Eustachian valve - 17%
- open foramen ovale (OOF) – 3.5%
- aneurysm of the interatrial septum - 4.7%
- borderline narrow aortic root - 7%
- borderline wide aortic root - 5.8%
With age, there is a decrease in the incidence of an enlarged Eustachian valve, prolapse of the pectineus muscles into the right atrium, mitral valve prolapse, closure of the patent foramen ovale, dilatation of the right atrioventricular orifice, asymmetry of the aortic valve leaflets, normalization of the length of the mitral valve chords and the diameter of the great vessels.
A change in the incidence of individual minor anomalies of the heart may be associated with the processes of reverse development of connective tissue structures (the Eustachian valve rudiments with age) or with an adaptive restructuring of the blood circulation - the load on the right ventricle decreases with age, on the left - increases.
Types of atrial septal aneurysm
Classification
Localization and form
Atria and interatrial septum:
- prolapsing valve of the inferior vena cava
- enlarged Eustachian valve (>1 cm)
- patent oval window
- atrial septal aneurysm
- prolapsing pectineus muscles in the right atrium
Tricuspid valve:
- displacement of the septal leaflet into the cavity of the right ventricle within 10 mm
- dilatation of the right atrioventricular opening (ring)
- tricuspid valve prolapse
Pulmonary artery:
- dilatation of the pulmonary artery trunk
- pulmonary valve prolapse
Aorta:
- organically narrow and wide aortic root
- dilatation of sinus of Valsalva
- bicuspid aortic valve
- asymmetry of the closure of the crescents of the aortic valve
- aortic valve cusp prolapse
Left ventricle:
- trabeculae or chords (transverse, longitudinal, diagonal)
- accessory papillary muscles
- S-shaped deformation of the interventricular septum with a systolic cushion in the outflow tract
- small aneurysm of the interventricular septum
Mitral valve:
- mitral valve prolapse
- ectopic attachment of chordae
- violation of the distribution of chords of the anterior and/or posterior valve
- "fluttering" chords
- additional and abnormally located papillary muscles
Complications and associated changes
- infective endocarditis
- calcification
- myxomatosis
- fibrosis of valve leaflets
- chord ruptures
- heart rhythm disturbances
Hemodynamic characteristics
- regurgitation and its degree
- presence of circulatory failure
- presence of pulmonary hypertension
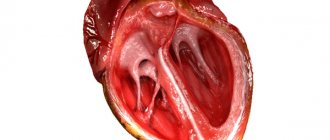
Mitral valve prolapse
Prolapse refers to an anatomical defect in the mitral heart valve. It is located between the left ventricle and the atrium. When the atrium contracts, it opens, which ensures the free flow of blood into the ventricle - and further, through the general blood flow. If a serious pathology of the mitral valve develops, its leaflets “bend”. In the worst case, the blood partially remains in the left atrium, which is fraught with unpleasant symptoms:
- heart pain;
- dizziness and shortness of breath;
- fainting;
- interruptions in heart function.
Typically, such symptoms are the exception to the rule, and minor mitral valve prolapse does not pose a threat to the life and health of the child. Severe prolapse is dangerous. It is manifested by severe deflection of the valves and more serious disturbances of cardiac activity (in the second and third degrees).
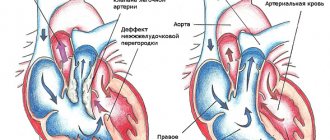
Patent foramen ovale (PFO)
Frequency in the population - 15%
The morphological anomaly is an oval window closed by a fold that is not adherent to the septum
EchoCG criteria
Gradual wedge-shaped thinning of the interatrial septum with interruption of the echo signal (as a rule, there is no sharp break as with an atrial septal defect).
A slight left-to-right shunt can be determined by the color circulation (there is a dependence of the discharge on the respiratory phase)
Pressure in the right side of the heart is within normal limits
Open oval window. A discharge of blood flow from left to right is visualized, the diameter of the jet is 4 mm. Epigastric section.
Algorithms for the management of patients with structural cardiac abnormalities in therapeutic practice
In clinical practice, primary care physicians quite often encounter patients who have various types of congenital structural abnormalities of the heart. We are talking about the so-called minor heart anomalies, which are not related to true congenital heart defects. These cardiac anomalies are based on genetic (primary) and non-genetic (secondary) disorders of the synthesis and/or breakdown of connective tissue proteins of varying severity. Due to these circumstances, most practicing doctors do not pay due attention to this category of patients and often regard the clinical manifestations that accompany minor cardiac anomalies as autonomic dysfunction.
The introduction of echocardiographic diagnostic methods into practical medicine and the improvement of echocardiographic imaging technologies in recent decades have made it possible to timely identify various types of congenital heart anomalies, which are not always harmless and, under certain conditions, can cause serious complications, even fatal.
Of the structural abnormalities of the heart in clinical practice, the most common are mitral valve prolapse (MVP), patent foramen ovale (PFO) and atrial septal aneurysm (ASA). Below we will focus on the most typical symptoms and clinical manifestations, possible complications and treatment tactics for minor cardiac anomalies, which will allow practitioners to choose the right tactics for passive or active monitoring of patients with varying degrees of severity of cardiac development defects.
Mitral valve prolapse
Currently, there is no uniform terminology and classification of PMC [1]. Primary MVP is usually distinguished: familial and non-familial (sporadic). Familial prolapse is based on dysplastic changes in the connective tissue of two pathomorphological forms: Barlow's disease or syndrome (myxomatous degeneration) and fibroelastin deficiency (FEN) (Fig.). These features are important for deciding on the nature of surgical correction—mitral valve repair or replacement. Secondary MVP is considered as a structural abnormality of the heart in hereditary connective tissue disorders (HCTD or connective tissue dysplasia (CTD)), such as Marfan syndrome, Ehlers-Danlos syndrome, and other monogenic conditions. In addition, the most common causes of secondary MVP are ischemic heart disease (against the background of dysfunction of the papillary muscles associated with ischemia), rheumatic attack (when the severity of the process subsides, prolapse may disappear), conditions accompanied by a decrease in the left ventricle and the annulus of the mitral complex (hypertrophic cardiomyopathy, pulmonary hypertension, atrial septal defect, dehydration, straight back syndrome, pectus excavatum), etc.
Recent data show that the prevalence of MVP in the general population is highly variable. When examining the young contingent, it occurs in up to 11–18% among athletes and 4.9–8.1% among people of military age. The most objective is the prevalence of MVP in the range of 0.6–2.7%, due to the introduction of new criteria for echocardiographic examination of the fibrous atrioventricular ring. Large values should be regarded as a consequence of overdiagnosis [2].
Complaints
Dysfunction of the autonomic nervous system in patients with MVP is characterized by a variety of subjective sensations. Stitching and aching pain in the precordial area is prolonged and not associated with physical activity. Rapid heartbeat and interruptions in heart function often occur at rest; a feeling of lack of air, “dissatisfaction” with breathing, difficult, “dreary” breathing characterize respiratory neurosis. Lability of blood pressure situationally, against the background of weather conditions, is transient in nature and is not accompanied by damage to target organs. Neurological symptoms are manifested by dizziness, migraine-like headaches, the development of presyncope and syncope, and impaired thermoregulation.
Inspection
The likelihood of clinically and hemodynamically significant MVP is extremely low in individuals who do not have external signs of systemic connective tissue involvement (SICT) and do not complain. Upon examination, patients usually have an asthenic physique, low body mass index, underdeveloped muscles, bone, skin and joint signs of DST. Characteristic skeletal changes are a funnel-shaped or keeled chest; changes in posture in the form of spinal deformity (kyphosis, scoliosis), straight back syndrome, flat feet, joint hypermobility with arthralgic syndrome often occur. Weakness of the vascular wall manifests itself in the form of varicose veins and weakness of their valve apparatus, hemorrhoids and varicoceles.
ECG changes
ECG changes in MVP have three options:
1) isolated inversion of T waves in limb leads II, III, AVF without ST segment displacement; 2) inversion of T waves in the limb leads and left chest leads (mainly in V5–V6) in combination with a slight shift of the ST segment below the isoline; 3) inversion of T waves in combination with ST segment elevation. To identify heart rhythm disturbances, daily ECG monitoring is optimal.
Echocardiography
According to echocardiography (EchoCG), MVP as a structural cardiac anomaly (SAC) is considered in the following cases:
- the presence of echocardiographic data corresponding to displacement of the leaflet(s) by more than 2 mm above the level of the fibrous ring of the mitral valve in the left atrium during systole;
- absence of signs of SIDS sufficient to make a diagnosis of MVP syndrome.
According to the recommendations of the AHA/ACC (American Heart Association/American College of Cardiology) of 2006, it is proposed to distinguish two options for mitral valve prolapse based on thickening of the leaflets in diastole:
- classic (myxomatous) MVP - with leaflet thickness > 5 mm;
- non-classical (non-myxomatous) - with a thickness < 5 mm.
Complications
Complications of MVP are accompanied by an unfavorable prognosis in 2–4% of cases and in almost 95–100% in the presence of myxomatous degeneration of the leaflets. These include:
- acute or chronic mitral regurgitation, due to rupture of the chordae tendineae and/or avulsion of the papillary muscle, leading to dysfunction and progressive dilatation of the left ventricle and left atrium;
- addition of infective endocarditis (moderate risk);
- thromboembolism, often cerebral, associated with thrombosis of myxomatous mitral valves;
- heart rhythm and conduction disorders;
- life-threatening arrhythmias, sudden cardiac death.
The clinical significance and complications of MVP as a structural cardiac abnormality have not been clearly defined.
Forecast
Risk stratification for MVP is based on assessing the severity of mitral regurgitation (MR), determining the thickness of the mitral valve leaflets and associated risk factors. Major (primary) risk factors include:
- mitral regurgitation ≥ II degree;
- myxomatosis ≥ 5 mm;
- ejection fraction <50%.
Minor risk factors are considered:
- mitral regurgitation degree I;
- myxomatosis < 5 mm (i.e. 3–4 mm);
- left atrium < 40 mm;
- “threshing door”;
- age > 50 years;
- atrial fibrillation.
A low risk of complications is assessed in the absence of myxomatosis and/or grade 0–1 mitral regurgitation; medium - in the presence of one large or 2 small risk factors; high – with 2 major or three or more minor risk factors; very high - with two large risk factors in people over 50 years of age against the background of chordal rupture or cardiomegaly with signs of chronic heart failure.
In terms of an unfavorable prognosis of cardiovascular events, auscultatory data, the depth of leaflet prolapse and the severity of endothelial dysfunction are also taken into account.
Therapeutic tactics for MVP
Treatment of patients with MVP can be medical or surgical, depending on the severity of mitral regurgitation.
Most patients with MVP do not need treatment in the absence of clinical symptoms, and they are recommended to lead a normal lifestyle. Asymptomatic patients with MVP without mitral regurgitation should undergo clinical evaluation every 3 to 5 years.
Patients with MVP and complaints associated with dysfunction of the autonomic nervous system (cardialgia, palpitations, interruptions in heart function, respiratory disorders) are advised to avoid stimulants such as caffeine, alcohol, smoking; Beta-blockers are recommended for drug therapy. There is convincing evidence of the positive effects of magnesium orotate.
In patients with systolic dysfunction and severe mitral regurgitation with an ejection fraction less than 60%, drug therapy should include beta-blockers, angiotensin-converting enzyme (ACE) inhibitors or sartans, and possibly aldosterone antagonists.
Antibacterial therapy for the prevention of infective endocarditis in MVP (intermediate risk) is not carried out. Annual sanitation of foci of infection is recommended (hygiene of the oral cavity and skin, urogenital tract), piercing and tattoos are prohibited, preference is given to peripheral catheters with their systematic replacement every 3-4 days. Performing any surgical intervention on the mitral valve places the patient at high risk for infective endocarditis. Along with nonspecific, a standard regimen of antibacterial prophylaxis is provided (amoxicillin at a dose of 2 g orally one hour before the intended intervention).
Patent foramen ovale
Next to MVP in terms of the frequency of cardiovascular complications among minor cardiac anomalies is a patent foramen ovale (PFO). In 1877, J. Cohenheim first suggested that the death of a young woman due to an ischemic stroke occurred as a result of the penetration of a blood clot through the LLC from the venous system into the arteries of the brain. Thus, PFO first became associated with the concept of paradoxical embolism (PE) and stroke [3].
The morphology of LLC is very diverse. Variability concerns the size, shape of the oval window, the thickness of its edges, the presence of a valve or its reduction to varying degrees; communication may be a winding “tunnel” passage. A patent oval window should be discussed after the first year of a child’s life.
Clinical manifestations in most cases are mild, and therefore this structural abnormality of the heart is detected extremely rarely. Symptoms at which one should suspect a valvular defective PFO (functioning) are the following:
- slight cyanosis of the lips or nasolabial triangle during coughing, physical activity, or crying in children;
- persistent predisposition to colds and inflammatory bronchopulmonary diseases;
- delayed physical development of the child, low tolerance to physical activity, especially in combination with symptoms of respiratory discomfort or respiratory failure;
- the occurrence of unexplained loss of consciousness, fainting, symptoms of transient cerebrovascular accident, especially in young people and patients with varicose veins and/or thrombophlebitis of the lower extremities and/or pelvis;
- minimal radiological signs of hypervolemia in the vascular bed of the lungs, a tendency to enlarge the right chambers of the heart (usually in older age);
- minimal electrocardiographic changes indicating an increase in the hemodynamic load on the right parts of the heart, especially on the right atrium, with complete or partial block of the right bundle branch.
Cyanosis of the skin and mucous membranes is a consequence of the discharge of venous blood into the systemic circulation and is associated with activation of the right-left interatrial shunt. Its occurrence is caused by the Valsalva phenomenon against the background of physical activity, straining when coughing and crying, and holding your breath. The danger of this abnormal discharge is associated with possible thromboembolic complications. Strokes associated with PFO are more often defined as cryptogenic (CI). The frequency of detection of PFO in CI is extremely high - from 24% to 66% of cases. However, the cause-and-effect relationship is still not so obvious [4].
Paradoxical venous embolism (PVE)—migration of a thrombus (rarely air or fat) from the venous system into the left atrium with subsequent embolism into the systemic circulation—is one of the main complications of PFO. Clinically, PVE can manifest as ischemic stroke (IS), transient ischemic attack (TIA) and, extremely rarely, infarctions of visceral organs (intestines, myocardium, spleen, retina) [5, 6].
In patients with PFO without right-to-left intra-atrial shunting, IS may develop due to the formation of a thrombus in situ (paradoxical embolism), either near the PFO, combined with an aneurysm of the interatrial septum, or this is facilitated by a tunnel-like form of communication.
Migraine with aura is the second most common clinical event in PFO after cerebrovascular accident. Trigger factors for the occurrence of migraine with aura during right-left shunting are associated with the potential for vasoactive substances to enter the cerebral bloodstream through interatrial communication, bypassing the lungs, where they should normally be destroyed [7]. Factors that trigger migraines include: stress, depression, poor diet, lack of sleep, certain foods (chocolate, citrus fruits, cheese, red wines), volatile compounds (perfume, exhaust fumes, tobacco smoke, etc.).
Diagnostics
For the initial diagnosis of a functioning PFO, in many cases, transthoracic echocardiography (TTEchocardiography) is sufficient. The exception is for high-risk individuals. These include: technical divers diving to depths of more than 30 m, submarine crews, fighter pilots and astronauts experiencing g-forces of several atmospheres.
The most informative method for diagnosing a functioning PFO is transesophageal echocardiography (TEE). To improve visualization of the shunt, TEE is complemented by contrasting the right parts of the heart with performing the Valsalva maneuver (inhalation and straining of the patient at the doctor’s command).
Risk stratification, prognosis of paradoxical embolism and cryptogenic stroke
Since 2014, risk stratification for PFO (along with MPP aneurysm (AMPP), MVP, mitral annulus calcification or mitral stenosis without atrial fibrillation (AF), etc.) is considered in the category of cardiac causes of IS as an option with low and/or, with certain conditions, with an average risk of cerebral cardioembolism.
The main risk factors for thromboembolic complications in PFO:
- young age (<55 years);
- family history of CI in patients with PFO;
- concomitant deep vein thrombosis of the lower extremities and pelvis;
- various invasive medical procedures.
A separate category of people at risk of CI may include young women with migraine pain with aura and patients with various thrombophilic conditions.
EchoCG predictors of CI associated with PFO are:
- tension during the Valsalva maneuver;
- large oval window size (> 4 mm);
- elongated Eustachian valve (UEV);
- spontaneous right-to-left shunt;
- positive saline contrast test and high degree of contrast;
- anatomical topography of the LLC, predisposing to embolization.
Therapeutic tactics for LLC
In the postnatal period, three main options for the course of PFO are possible: spontaneous closure (holes up to 4–5 mm in size can close), maintaining a constant size, and increasing the defect over time (in older people).
Asymptomatic PFO without risk factors is not an indication for prescribing disaggregant and anticoagulant therapy. The feasibility of their use is discussed from the perspective of secondary prevention of stroke in patients with PFO. If heart failure develops, standard symptomatic therapy is recommended.
With the development of overload of the right parts of the heart, heart failure, including in persons of certain specialties (pilots, cosmonauts, submarine crew members, divers, scuba divers, caisson workers), it is justified to close the hole surgically, often endovascularly using an occluder.
Prevention of recurrent thromboembolism in patients with PFO who have suffered a cryptogenic stroke or TIA may be limited to the prescription of antiplatelet agents in the absence of standard indications for the prescription of anticoagulants, as well as to certain clinical situations.
Atrial septal aneurysm (ASA)
AMPP is a primary developmental anomaly and anatomically represents a pronounced protrusion of excess tissue in the projection of the oval fossa. Up to 70% of aneurysms have one or more holes through which blood is shunted, and such options are considered as a combination of an aneurysm with small defects of the aneurysm. Secondary AMPP is caused by diseases that cause increased pressure in the right or left atrium. In most cases, AMPP proceeds safely, without hemodynamically significant changes and clinical manifestations. Studies have shown a significant increase in the risk of stroke in the presence of AMPP and LLC. Moreover, the risk ratio was even greater with AMPP than with LLC, increasing maximally with their combination [8].
Aneurysm of the MPP, as well as PFO, can be classified as a low-risk factor, and under certain conditions - medium-risk.
The risk of PE increases significantly in the presence of a highly mobile bladder (promotes a wider opening of the oval window or deterioration of the systolic function of the left atrium), leading to a slowdown in blood flow and an increased risk of thrombus formation.
Treatment tactics for aneurysm of the interatrial septum
Antiplatelet therapy is not indicated for patients with isolated AMPP.
With transcatheter closure of PFO combined with AMPP, a significant decrease in the amplitude of its fluctuations and a decrease in the likelihood of repeated embolic episodes were noted. However, AMPP is associated with a higher incidence of residual shunts after percutaneous PFO closure, even when using occluders of the maximum diameter.
Conclusion
The presented data clearly demonstrates the fact that structural abnormalities of the heart, under certain conditions, can pose a real threat to the health and life of patients. The frequent subclinical course of structural abnormalities of the heart makes it difficult to detect them in a timely manner and requires the focused attention of practicing physicians in assessing the phenotypic features of the structure of the musculoskeletal system and connective tissue, which can serve as markers of hidden defects in cardiac development. At the same time, it should be remembered that the main method for detecting minor anomalies is the echocardiographic method, which, with technological progress, is undergoing significant changes. Consequently, the diagnosis of anatomical anomalies is an integrative process, which is based on a close tandem of clinical thinking and instrumental research methods. Early detection of structural abnormalities of the heart and the choice of the correct tactics of passive or active monitoring of patients with varying degrees of severity of cardiac development defects will help preserve the health or even life of patients.
Literature
- Supranational (international) recommendations for structural abnormalities of the heart // Medical Bulletin of the North Caucasus. 2020. T. 13. No. 1–2.
- Malev E. G. Prevalence, pathogenetic mechanisms and features of management of patients with mitral valve prolapse. 2014.
- Onishchenko E. F. Patent foramen ovale and stroke in clinical practice. St. Petersburg: ELBI-SPb, 2005.
- Heart RG, Diener HC, Coutts SB et al. Cryptogenic Stroke/ESUS International Working Group. Embolic stroks of undetermined source: the case for a new clinical construct // The Lancet Neurology. 2014; 13 (4): 429–438.
- Cramer SC, Rordorf G, Maki JH et al. Increased pelvic vein thrombi in cryptogenic stroke: results of the Paradoxical Emboli frome Larde Veins in Ischemic Stroke (Pelvis) study // Stroke. 2004; 35 (1): 46–50. https://doi.org/10.1161/01.str.0000106137.42649.ab.
- Scarvelis D., Wells PS Diagnosis and treatment of deepvein thrombosis // Canadian Medical Association Journal. 2006; 175(9):1087–1092. DOI: 10.1503/cmaj.060366.
- Caputi L., Usai S., Carriero MR et al. Microembolic Air Load During Contrast Transcranial Doppler: A Trigger for Migraine With Aura? // Headache: The Journal of Head and Face Pain. 2010; 50(8):1320–1327. https://doi.org/10.1111/j1526-4610.2010.01621.x.
- Hara H., Virmani R., Ladich E. et al. Patent Foramen Ovale: Current Pathology, Pathophysiology, and Clinical Status // Journal of the American College of Cardiology. 2005; 46(9):1768–1776. https://doi.org/10.1016j.jacc.2005.08.038.
E. A. Barabanova, Candidate of Medical Sciences A. A. Arakelyants, Candidate of Medical Sciences T. V. Zaugolnikova, Candidate of Medical Sciences T. E. Morozova1, Doctor of Medical Sciences, Professor
Federal State Autonomous Educational Institution of Higher Education First Moscow State Medical University named after. I. M. Sechenov Ministry of Health of Russia, Moscow
1 Contact information
DOI: 10.26295/OS.2020.21.50.002
Algorithms for the management of patients with structural abnormalities of the heart in therapeutic practice / E. A. Barabanova, A. A. Arakelyants, T. V. Zaugolnikova, T. E. Morozova For citation: Attending physician No. 2/2020; Page numbers in the issue: 10-14 Tags: mitral regurgitation, markers of hidden defects, congenital anomalies
Buy an issue with this article in pdf
Enlarged (elongated) Eustachian valve
Eustachian valve / Eustachian valve / valve of the inferior vena cava (valvula venae cavae inferioris) - is a fold of the endocardium, which after the neonatal period does not exceed 1 cm in length or is completely rudimentary.
Minor cardiac anomalies include cases of Eustachian valve enlargement of more than 1 cm and its prolapse.
In population studies, it occurs in 0.2% of those examined.
Among minor cardiac anomalies it is 17.6-25.9%
Prolapse of the Eustachian valve - 1.0-4.7%
Video 1. Elongated Eustachian valve.
Causes of minor anomalies of heart development in a child
Why do these anomalies occur? As already mentioned, the development of heart anomalies is based on changes in the properties of the connective tissue of this organ. The main cause of connective tissue dysplasia of the heart is a genetically determined defect in the synthesis of collagen type 111 (this is a protein used as the main building material of the body). That is, congenital structural features of the heart are inherited by the child, especially from the mother. Also important here are unfavorable environmental conditions, stress, insufficient intake of vitamins from the pregnant woman’s diet, use of toxic substances during pregnancy (drugs, alcohol, nicotine), oxygen starvation of the fetus and all those factors that can have a negative impact on the fetus during intrauterine development. .
Microanomalies of the right atrium
The Chiari network is a fibrous structure extending from the crest border or tubercle inferiori to the eustachian or basal valve. The frequency among congenital anomalies is 4%.
Prolapse of the pectineal muscles in the cavity of the right atrium - in the form of thin thread-like and mobile structures in the area of the wall of the right atrium or appendage. Frequency among congenital anomalies - 9.7%
Causes of pathology in children, adolescents and adults
The MARS anomaly may be genetically determined. The main “culprit” of connective tissue weakness is the synthesis of defective collagen fibers.
Such conditions are rarely limited to just the structure of the heart, but extend to many internal organs. For example, with Marfan syndrome, the limbs and chest are deformed, vision is impaired, and an aortic aneurysm forms.
Also important factors that disrupt the formation of the cardiovascular system are:
- taking alcohol, drugs and medications,
- smoking,
- poisoning,
- viral infections,
- irradiation,
- stress,
- poor nutrition – lack of protein and vitamins,
- lack of oxygen in the blood due to anemia,
- diabetes,
- thyroid disease.
Minor anomalies of the aorta
A borderline narrow aortic root is the presence of low values of aortic diameter (from the 3rd percentile and below the distribution curve), with the absolute absence of both signs of stenosis (including Dopplerography) and ECG signs of left ventricular hypertrophy or left-sided overload. The incidence among minor cardiac anomalies is 7-10%
Primary EchoCG criteria:
- aortic diameter less than 20 mm
- absence of a significant pressure gradient between the aorta and left ventricle
Borderline wide aortic root - the presence of increased values of aortic diameter (from 90 percentile or more of the distribution curve). The incidence among minor cardiac anomalies is 5.8-9%.
Dilatation of the sinuses of Valsalva - the presence of dilatation of the sinuses from 3 to 7 mm (usually non-coronary, less often right and left). Occurrence in the population is 0.26%, among minor cardiac anomalies - 27.5%
Asymmetry of the aortic valve leaflets - the presence of unevenly developed and located leaflets (detected in the parasternal position along the short axis). Occurrence among MAS - 8.2%
Aortic valve prolapse is a diastolic protrusion towards the left ventricular outflow tract (more than 1 mm from the line of the annulus fibrosus). Occurrence among MAS - 6.6%
Symptoms
A pediatrician may suspect a disease in a child during a routine examination and examination of the child, paying attention to a systolic murmur during cardiac auscultation.
In most cases, the additional chord of the left ventricle does not bear any functional load on the heart and does not interfere with its normal functioning. For many years, this minor anomaly may not be detected, because it is not accompanied by any special symptoms. A pediatrician can listen to a systolic heart murmur in a newborn, which is detected between the third and fourth ribs to the left of the sternum and does not in any way affect the functioning of the heart.
During intensive development, when the rapid growth of the musculoskeletal system significantly outstrips the growth rate of internal organs, the load on the heart increases, and the additional chord may make itself felt for the first time. Your child may experience the following symptoms:
- dizziness;
- rapid or unmotivated fatigue;
- psycho-emotional lability;
- cardiopalmus;
- pain in the heart area;
- heart rhythm disturbances.
The same clinical manifestations can be observed with multiple abnormal chords of the left ventricle. Most often, such symptoms appear during adolescence. In the future, they can completely disappear on their own, but sometimes they remain in adulthood.
When symptoms appear, the child is required to undergo ECHO-CG, ECG and 24-hour Holter monitoring. These studies will allow the doctor to determine the presence or absence of hemodynamic disturbances. If the additional chord is “hemodynamically insignificant,” then the anomaly is considered safe, and the child only requires follow-up with a cardiologist. With a “hemodynamically significant” diagnosis, the patient is advised to observe, adhere to certain restrictions and, if necessary, treatment.
Abnormally located trabeculae, false chords
From the history
The first description is by W. Turner (1893) based on autopsy data
The first term is “moderator band” (1893)
The first classification is MCLam et al. (1970), the term “false chordae tendineae”, i.e. false chord
The first description according to echocardiography data in 1981.
Prevalence of abnormally located trabeculae, false chordae
- according to population studies up to 17%
- autopsy data up to 16%
- according to echocardiography 2.3-68%
- in children with manifestations of CTD in 94%
Video 2. Open oval window. A blood flow discharge from left to right is visualized. Jet diameter 4 mm. Epigastric section.
What is a false chord and anomalous trabecula?
The true notochord is a fibrous cord connecting the papillary muscle to the valve.
False chord is a fibromuscular or fibrous cord connecting the papillary muscles to the wall of the left ventricle or to each other.
Normal trabecula is a muscle cord tightly adjacent to the ventricular endocardium.
Abnormal trabecula is a muscular or fibromuscular cord loosely adjacent to the ventricular endocardium.
Violation of the distribution of chords - their predominant attachment to the base of the valve and, to a lesser extent, in the area of the body or free edge (in systole additional echo signals are located in the outflow tract of the left ventricle)
Lengthening of the chords - additional thin, linear echo structures, performing large-amplitude diastolic movements
Ectopic attachment of chords - attachment of the chord from the wall of the left ventricle to the mitral valve leaflet.
False chords
Quantity:
- single
- multiple
Direction:
- transverse
- diagonal
- longitudinal
Level:
- apical
- median
- basal
Aneurysm of the interatrial septum
The wall between the left and right atrium can sometimes be curved. It protrudes to the side - to the right or to the left. Most often, the cause of its development in children is a hereditary factor. Intrauterine infections can also provoke the development of an aneurysm of the interatrial septum. At each age, different clinical manifestations of this anomaly can be distinguished:
- 1-3 years. Children lag behind in physical and mental development and often suffer from viral infections. During an objective examination, doctors often detect right ventricular hypertrophy with signs of pulmonary circulation overload;
- older age (12-15 years). Children and adolescents are stunted and do not tolerate physical activity well. Symptoms include: heart rhythm disturbances, weakness, pain, pale skin.
To avoid rupture of the aneurysm, it should be regularly monitored and modern diagnostic procedures performed.
Microanomalies of the papillary muscles
This anomaly includes changes:
- forms
- quantities
- location
According to the autopsy:
- Two papillary muscles - 65.2%
- Three papillary muscles - 13%
- Four papillary muscles - 4%
- Five papillary muscles - 4.4%
- Six papillary muscles - 4.4%
Tags: abnormal trabecula, Abnormally located trabeculae, heart disease, true chord, false chords, macroanomalies of the heart, minor cardiac anomalies, MAS, Microanomalies of the papillary muscles, LLC, Patent foramen ovale, right atrium, mitral valve prolapse, pulmonary artery dilatation, heart, Chiari network, Chiari network, trabecula, Elongated Eustachian valve