Diseases of cardiac structures in children occur in 3-5% of cases of the general young population. Diagnoses are not equally dangerous; to say something specific requires a thorough, comprehensive diagnosis.
A heart murmur in a child is a pathological sound that is detected during auscultation between functional moments: systole (heart contractions) and diastole (relaxation of a muscle organ).
It's not always about illness. In adults, changes recorded during auscultation (listening) are clearly of an abnormal nature.
Heart murmurs at an early age require additional instrumental diagnostics to identify their genesis. At a minimum, an ECG and echocardiography are performed.
The clinical picture, if present, is nonspecific and indicates one or another abnormality.
Therapy is conservative for disorders not associated with anatomical changes, surgical therapy as part of the treatment of congenital or acquired defects in early years.
Types of noise
The classification is carried out on two key grounds. The first is by type of deviation from the conventional norm.
Accordingly, they distinguish:
- Functional noise (physiological, FSH in abbreviated form). If in adults an objective symptom always indicates anomalies or defects, in childhood this is not an axiom, but in 97% a variant of the norm.
Moreover, in approximately half of the cases, or even more, in young patients extraneous sounds are detected during auscultation.
In the 1st, 2nd and 3rd years of life, the reason lies in the restructuring of the cardiovascular system, adaptation to existence outside the womb.
From the age of 4, a connection is found with maturation, body growth, and then with puberty. Conventionally, childhood ends at the beginning of puberty, that is, by the age of 12-13, then the noises disappear.
FFS in a child is not a diagnosis, but a functional heart murmur, a sound heard during auscultation.
- Pathological noises (organic). They account for the other half of the recorded situations.
They arise as a result of the influence of many factors. Including defects, congenital and acquired.
It will not be possible to delimit the conditions “by eye”. Instrumental diagnostics are required. You can assume there is a problem based on the symptoms.
Attention:
Within functional murmurs there is no clinical picture (symptoms). It may not be present in the disease, therefore it is not a reliable differential criterion.
It is possible to subdivide on a different basis. It concerns the moment when an anomalous sound occurs. Then they tell a couple more violations.
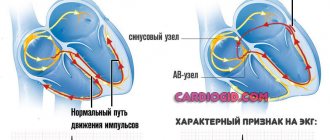
There are two normal heart sounds:
- The first is systolic. Occurs when cardiac structures contract and atrioventricular valves close. Normally, after it and until the moment of relaxation there are no symptoms on auscultation.
Murmurs most often indicate defects, such as mitral, aortic, valve prolapse, their defects and other phenomena.
- The second tone is diastolic. It is detected after the blood is pushed out into the systemic circle, when it returns to the pulmonary artery.
In this case, disturbances in auscultation are detected due to functional disorders (in an abnormal sense). For example, as part of the experience of myocardial inflammation and other issues.
In some cases, a violation of all tones is detected at once, then the murmur is recorded twice in one complete contractile cycle of the heart.
Classifications are widely used by doctors as part of the initial examination and development of treatment tactics.
Systolic heart murmur in a child is more indicative. But it does not give an unambiguous answer to the questions “what is it” and “why did it arise.” Electrocardiography and ECHO-CG put an end to it.
If necessary, additional methods, such as MRI, X-ray.
Heart murmur
One of the common signs of heart damage is heart murmurs. Heart murmurs are currently recorded in more than half of all children born. There is an opinion that all children have a heart murmur at some point in their growth, but the causes vary. And although in most cases the murmur does not indicate the presence of organic heart pathology, it must be treated with great attention. Such children are subjected to additional examinations and observed by a cardiologist.
Murmurs are divided into systolic and diastolic. They can be organic or functional in origin. The former are characteristic of anomalies in the development of the heart; the causes of functional murmurs can be different. It is traditionally believed that systolic murmur is more characteristic of its functional nature. Diastolic murmurs in children in most cases have an organic origin (cause) and occur with insufficiency of the aortic and pulmonary valves; stenosis of the left and right atrioventricular openings; pathological discharge of blood in diastole: aortopulmonary septal defect, open aortic duct, etc.
Noise also differs in volume, duration, timbre, zone of maximum localization and area of preferential conduction.
For clarity, data on the characteristics and causes of functional noise in children and adolescents can be displayed in the table:
| Noise | Approximate age | Time characteristic | Origin |
| Murmur of peripheral pulmonary stenosis | Newborn | Systolic ejection murmur | Pulmonary artery bifurcation |
| Vibrating Still noise | 3–8 years | Systolic ejection murmur | Unknown |
| Carotid murmur | 3–8 years | Systolic ejection murmur | Carotid arteries |
| Venous murmur – “spinning top noise” | 3–8 years | Continuous | Jugular and superior vena cava |
| Pulmonary flow murmur | 6–18 years | Systolic ejection murmur | Pulmonary valve |
But few parents know that the murmur can accompany minor structural abnormalities in the development of the heart that do not significantly affect its functioning, or reflect significant disturbances in blood flow associated with congenital heart disease.
With the introduction of ultrasound examination of the heart (echocardiography) into widespread practice, it became clear that for the occurrence of murmur, some anatomical reason is necessary, that is, they are almost all “organic”. Therefore, it is currently considered more correct to divide noises into “innocent” and “pathological”.
Parents need to know: functional noises can also occur in practically healthy children at different ages.
Advertising
Causes of pathological noises
We can name congenital and acquired diseases that provoke abnormal auscultation results. The first are represented by tissue abnormalities.
- Mitral valve insufficiency. It provokes regurgitation, that is, the flow of blood from the left ventricle back into the atrium. Which, of course, should not be normal.
The defect provokes insufficient trophism (nutrition) of all tissues of the human body. A meager amount is released into the aorta and reaches the systems.
The brain, liver, kidneys, the heart itself, and the digestive tract are affected. Serious hemodynamic deviations, and then complications, do not develop overnight.
It usually takes several years to progress, but variations are possible.
- Insufficiency of the aortic and tricuspid valves. Provokes disorders of normal blood flow and nutrition of all organs and systems. The mechanisms are associated with improper circulation of fluid tissue throughout the body.
- Defects of the interatrial septum.
These deviations from the norm are of genetic origin in 40% of cases or slightly more. That is, the basis of etiology is mutation.
This develops in the early periods, at the beginning of the first trimester, when the heart and blood vessels are just being formed.
Isolated disorders of the functioning of a muscular organ practically do not occur. Much more often we are talking about systemic chromosomal abnormalities. A classic example is Down syndrome. And there can be many more of them.
Another likely option is spontaneous intrauterine development disorders. The reason usually lies in illnesses suffered by the mother or negative factors affecting the fetus during the gestational period. For example, nicotine (smoking), alcohol, drugs.
Uncontrolled use of drugs, especially hormonal ones, has an impact; drugs based on synthetic progesterone are popular, which mothers take without permission, without thinking about the consequences.
High background radiation, poor diet with an excess of harmful substances (for example, salts of nitrous acids - nitrates). These factors must be taken into account during pregnancy in order to reduce risks to a minimum.
Acquired diseases are numerous. But in children they are still relatively rare.
- Rheumatism. Autoimmune pathology. Accompanied by regular recurrent inflammation of cardiac structures and myocardium.
It is not completely treatable, therefore the essence of therapy is to prevent exacerbations and relieve symptoms with the help of glucocorticoids and immunosuppressants.
- Infectious processes. Occurs in a relatively small number of cases. They are represented by inflammation of the myocardium itself (myocarditis), the pericardial sac, and other structures. Combined processes are possible.
Outside the main source of bacterial damage, deviation almost never occurs. Therefore, in all situations, third-party disorders are found: tonsillitis, caries, chronic tonsillitis, laryngitis, pharyngitis and other variants, as well as arthritis of infectious origin.
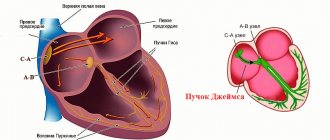
The list is incomplete. Other abnormalities are also possible, including collagenosis, proliferation of connective tissue, conduction disorders of special structures of the heart (bundle of His), arrhythmias, but this is most likely a consequence.
Because these diseases themselves do not arise spontaneously. The origin factor must be sought higher.
- It is extremely rare that the culprit is a heart attack. In children, the development of such a formidable condition is pure casuistry, an accident.
- An equally infrequent “guest” is chest injuries with damage to the heart. The ribs protect the internal organs well, and the intensity of the impact should be extremely high.
The classic version is a traffic accident with a sternum fracture and severe bruises. Abnormal sounds are detected after treatment.
- The causes of heart murmurs in a child also occur as a result of iatrogenic (medical) factors. As an example, after surgical treatment with endovascular or classical open access, examination for other pathologies (involves catheterization, penetration into the vascular bed and heart).
Indirectly, abnormal sounds can be caused by hypertension, changes in blood vessels as a result of vasculitis (inflammation of the internal lining of the arteries), atherosclerosis (found in 0.7-1.2% of cases, usually against the background of metabolic disorders, Itsenko-Cushing's disease).
The factors are varied and not always obvious. It makes sense to carefully examine a young patient.
Causes of heart murmurs in children
Heart murmurs in children are most often associated with certain body conditions and developmental disorders. Conventionally, the reasons can be divided into several groups:
- Deviations in the development of heart valves:
- stenosis of the mitral valve and aortic valve (its anatomical narrowing);
- regurgitation of the valves described above (backflow of blood);
- insufficiency or stenosis of the tricuspid (three-leaf) valve;
- pulmonary artery stenosis.
- Presence of abnormal holes:
- patent ductus arteriosus (the presence of an additional vessel connecting the pulmonary artery and the aorta);
- the presence of pathological holes between the chambers: ASD (atrial septal defect) and VSD (ventricular septal defect).
- Pathology of the myocardium (heart muscles):
- congenital changes – hypertrophic cardiomyopathy;
- acquired vices. Rarely encountered in childhood. These include myocardial infarction and heart failure.
- Other acquired and hereditary diseases:
- endocarditis – inflammation of the lining of the heart that forms the valves;
- Tetralogy of Fallot is a complex combined cardiac defect that combines damage to both the heart muscle and the valve apparatus;
- additional chord located in the left ventricle;
- Coarctation of the aorta is a narrowing of its lumen. Most often, the vessel is affected segmentally;
- Myxoma of the heart is a tumor (benign) located in one of the atria. Most often it grows on a stalk attached to the interatrial septum;
- hypoplastic left heart syndrome (HLHS) – insufficiency of development of all elements of the left atrium and ventricle;
- Ebstein's anomaly - accounts for about 1% of all heart defects. It is a displacement of the tricuspid valve leaflets into the cavity of the right ventricle.
- Functional reasons. These include the appearance of noise due to disturbances in the speed of blood flow, the occurrence of anemia, etc...
Symptoms
The clinical picture is absent in the case of functional heart murmurs. Therefore, it is not always possible to detect a deviation (relatively speaking); more often it is an accidental discovery, which becomes the reason for an in-depth study.
Unfortunately, this is not a reliable diagnostic sign. Because asymptomatic course of many pathologies is possible.
At an early stage, they manifest themselves in an objective way: changes in heart sound, blood pressure, and frequency of contractions of a muscle organ.
Characteristic manifestations at pronounced stages or with a clear course of the primary pathological process:
- Change in skin color. Quite an informative point; with a thorough assessment, you can say a lot of important things can be learned.
Thus, cyanosis of the upper and lower extremities indicates aortic stenosis (can be an acquired disorder or congenital coarctation), a change in the color of the fingertips indicates heart failure, etc.
The classic sign is a combination of blue discoloration of the nasolabial triangle and general pallor of the skin throughout the body with the formation of a visible vascular pattern.
- Tachypnea or increased respiratory rate per minute. In the early stages of the pathological process, it is detected under intense mechanical stress. During physical education or active play.
As the disease progresses, sub- and then decompensation occurs, with shortness of breath remaining at complete rest.
This is a negative sign. It indicates a general disorder, the beginning of severe organic changes in the lungs and the heart itself. Most likely, the brain is also affected.
This will be indicated by neurological symptoms: pain in the occipital, frontal, temporal regions, vertigo with the inability to navigate in space.
- Cough. Paroxysmal, unproductive. Develops spontaneously in later stages. In the early stages - in response to intense physical activity.
- Peripheral edema. The legs and feet suffer first. Then the whole body, which indicates the decompensated stage of the pathological process.
In rare cases, angina-type chest pain is possible.
It develops when the coronary arteries are damaged, which in itself is quite rare in children. Prevalence is about 6.5-7% of the total number of young patients.
As you can understand logically, these are symptoms of non-heart murmurs, which in themselves are considered a manifestation and not a disease. We are talking about the clinical picture against the background of a primary disorder, which also causes disturbances in auscultation.
Prevention of complications
Preventing the occurrence of heart murmurs implies the prevention of cardiovascular diseases, which must begin before the birth of the child. So, it is important for a pregnant woman to maintain a healthy lifestyle, eat healthy, not be nervous and not abuse alcohol and nicotine. It is important to regularly see a gynecologist and undergo all prescribed examinations, and consult a geneticist.
At the birth of a child, preventive measures include the following:
- Timely treatment of any inflammatory diseases.
- Consumption of vegetables, fruits and vitamins.
- Limiting fat intake.
- Adequate physical activity.
- Monitor the amount of iron in the blood.
Thus, maintaining a healthy lifestyle and proper nutrition is often quite sufficient to prevent many diseases associated with heart murmurs in children.
Diagnostics
Conducted by a cardiologist. Usually in an outpatient setting, hospitalization is required only in cases of severe health problems, in order to eliminate unnecessary risks for the child. The examination should be carried out quickly, but thoroughly.
Approximate list of events:
- Oral questioning of the patient. If a young patient is not yet able to speak, questions are asked to the parents. It is important to identify symptoms, objectify and formulate a clinical picture in order to put forward hypotheses regarding the origin of the disorder.
- Anamnesis collection. Previous illnesses, the course of childbirth, pregnancy in the mother, family history, and other points.
- Actually auscultation. Allows you to state a fact, determine the type of noise, the stage of its development.
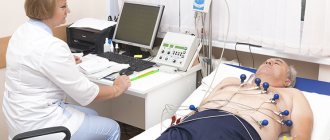
- Electrocardiography. Used for early assessment of functional disorders, arrhythmias, and some defects.
- Echocardiography. Essentially this is an ultrasound of cardiac structures. Used as part of tissue imaging. Makes it possible to detect anatomical defects.
- MRI. The most informative diagnostic path. Appointed in controversial cases.
- Blood pressure measurement. Within the framework of pathological processes, a stable increase in the tonometer indicator is observed. Once the disease reaches a certain point, a drop in level is detected. We are talking about sub- or decompensation.
- Daily monitoring as needed. Makes it possible to evaluate heart rate and blood pressure over a 24-hour period.
- Angiography. Not always.
- Chest X-ray.
- Blood tests. General and biochemical with an expanded picture of fatty structures (high and low density lipoproteins, atherogenic index), also, as needed, the level of thyroid hormones and adrenal cortex.
Doctors from the very beginning proceed from the possible pathogenic origin of the disorder.
FSS in a child is not a diagnosis, but an auscultatory sound, a functional heart murmur. They are normal and do not require treatment. Around the age of 12-13, everything should fade away on its own. Diagnostics puts an end to the issue.
When is it necessary to be examined?
Very often, during the first examination of a newborn, the child is diagnosed with a “heart murmur” without deciphering its nature. For most parents, such a diagnosis already sounds like a death sentence. But don't panic right away. Any heart murmur requires clarification of the cause of its occurrence, which means that the child needs to be examined and observed by a cardiologist. Don't wait for it to "go away on its own" (it may not happen). Currently, the main method for studying the functioning of the heart is ultrasound - echocardiography (echo-CG).
An electrocardiogram (ECG) will help to detect heart problems in a timely manner. An echocardiogram will complement the study by revealing the cause of the abnormalities.
When a child with a “functional” heart murmur continues to be monitored by a pediatric cardiologist or cardiorheumatologist for many months (or even years), but does not undergo an ultrasound examination of the heart, the situation can only be compared to sitting on a time bomb. After all, without conducting this study, which is common in modern times, one cannot be sure that a serious pathology of the cardiovascular system is not hidden in a child under the guise of “functional” noise. There is hardly a doctor who would risk ruling out heart disease with 100% certainty without conducting a special study. At the same time, it will not be difficult for an experienced diagnostician-instrumentalist to clarify (confirm or refute) a presumptive diagnosis in a matter of minutes.
Treatment
Organic noises require therapy. It can be conservative or surgical. Depends on the original cause of the violation.
The medicinal technique is based on the use of several groups of drugs:
- Antihypertensive. Reduce blood pressure (ACE inhibitors, beta blockers, calcium antagonists, mild potassium-sparing diuretics, for example, Veroshpiron).
There are a huge number of names of funds. However, it is impossible to choose a secure scheme on your own. Therefore, there is no point in clarifying the names.
If used incorrectly, heart failure and renal dysfunction may occur.
- Glycosides. Normalize myocardial contractility. Used in short courses, they restore blood flow. But this is a temporary measure in most cases.
Classic remedies - Digoxin, tincture of lily of the valley. If taken incorrectly (for a long time) or if dosages are not observed, fatal side effects and complications are likely.
It is also possible to use antiarrhythmics (Amiodarone), vitamin-mineral complexes and products based on microelements, magnesium and potassium (Asparkam and analogues).
As part of emergency relief of high blood pressure, Capoten is used in the amount of 1/6 - ¼ of the tablet. It is important that there is no sharp drop in the tonometer reading.
Surgical therapy is indicated in several cases: congenital defects of the heart itself (chambers, valves), blood vessels (type of fusion, blockage, anomalies of shape, anatomical development).
There are two ways to access the changed area (surgical field):
- Endovascular. Low-traumatic, by making pinpoint incisions and introducing surgical instruments. Requires a highly qualified treating specialist, special expensive equipment, and is not possible in all cases, only with small sizes of the altered lesion or insignificant volumes of intervention.
- Open access. In other situations.
The essence of the treatment is to eliminate the narrowing of the artery (ballooning or stenting, mechanical expansion), restore anatomical integrity in case of tissue abnormalities (plastic) or prosthetics (especially often with lesions of the valves, when other methods do not make sense).
With a competent approach, it is possible to achieve a complete cure. In the future, it is recommended to take medications for some time to normalize the rheological properties of the blood, and, if necessary, also to lower blood pressure.
Antiphospholipid syndrome in children
Antiphospholipid syndrome (APS) is a systemic autoimmune disease with arterial or venous thrombosis of various localizations, with miscarriage (spontaneous abortions, miscarriages, intrauterine fetal death) and with a high titer of pathogenetically significant antibodies to phospholipids (PL), such as antibodies to cardiolipin (CL). ), antibodies to lupus anticoagulant (LA) and antibodies to cofactor proteins (prothrombin, protein C, protein S, annexin V, prostacyclin and beta2-glycoprotein-I (beta2-GP-I) [13].
The study of APS began in 1907 with Wasserman’s development of a laboratory method for diagnosing syphilis. APS was first described in 1986 by English rheumatologist Hughes GRV. Since that time, intensive study of the pathophysiological and clinical features of APS has begun. The diagnostic criteria for APS, which were formulated in October 1998 at the VIII International Symposium, are divided into clinical and laboratory (
.).
Clinical criteria for APS:
- Vascular thrombosis
One or more clinical episodes of arterial, venous or small vessel thrombosis, in any tissue or organ. Thrombosis must be confirmed by Doppler ultrasound or histological examination, with the exception of superficial venous thrombosis. In histological examination, thrombosis should be represented by significant changes in the vascular wall of a non-inflammatory nature.
- Diseases of pregnant women
- a) One or more unexplained deaths of a morphologically normal fetus at or after the 10th week of normal pregnancy, with normal fetal morphology documented by ultrasound scanning or direct examination of the fetus, or
- b) One or more cases of preterm delivery of a morphologically normal fetus at or before 34 weeks of gestation due to severe preeclampsia or eclampsia, or severe placental insufficiency, or
- c) Three or more unexplained consecutive abortions before 10 weeks of pregnancy. Moreover, without pathological or anatomical abnormalities and/or hormonal disorders in the mother, and chromosomal causes should be excluded in the father and mother.
Laboratory criteria for API:
- Moderate or high levels of IgG and/or IgM anti-CL antibodies in the blood in two or more studies obtained at least 6 weeks apart, measured by standard and enzyme-linked immunosorbent assay (ELISA) for beta2-GP-I-dependent anti-CL antibodies.
- Positive test for the presence of lupus anticoagulant in plasma in two or more studies obtained at least 6 weeks apart [10].
Definite APS is diagnosed if at least one clinical and one laboratory criterion is present [5]. Unfortunately, these criteria were developed for diagnosing APS in adults and do not take into account the characteristics of children.
Detection of APS in pediatrics has important prognostic significance, since it implies a high risk of thrombosis, and also determines the course and outcome of the underlying disease. Associations between the presence of a circulating anticoagulant and vascular thrombosis in children were first noted in 1972 in children with systemic lupus erythematosus (SLE) [10].
Since 2000, the official website “European Forum of Antiphospholipid Antibodies” was created, where children with antibodies to FL are registered. Based on this database, a working classification of APS in children was published in 2006.
The diagnosis of APS for a child is reliable if there is a clinical criterion and at least one laboratory criterion.
It should be noted that when APS is suspected in children, an important criterion for making a diagnosis is family history, the presence in relatives of:
- rheumatic diseases;
- recurrent strokes (especially before the age of 50);
- recurrent heart attacks (especially before the age of 50);
- recurrent thrombophlebitis;
- miscarriage, eclampsia and preeclampsia.
In the studied groups of patients with APS, APS-associated signs of the disease were identified: thrombocytopenia, migraine or migraine-like headaches, nosebleeds, livedo reticularis, epilepsy, heart valve disorders, aseptic bone necrosis, chorea (hyperkinesis) and arterial hypertension. These manifestations are not clinical criteria, but their presence in combination with antibodies to FL is an important diagnostic factor for children with APS [14].
There are several clinical variants of APS in both children and adults:
- Primary APS (PAPS) - develops in individuals without autoimmune diseases.
- Secondary APS (SAPS) develops in patients with rheumatic and autoimmune diseases, with malignant neoplasms, when using a number of medications (hormonal, contraceptive, psychotropic substances, Novocainamide, high doses of interferon alpha), infectious diseases (herpes virus infection, mycoplasmosis).
- Catastrophic APS - multisystem, predominantly organ thrombosis at the level of the microvasculature with a high titer of antibodies to FL, disseminated intravascular activation with thrombosis in vessels with a small diameter, multiple organ damage to the body. The diagnostic criterion for catastrophic APS in children is the involvement of at least three systems of the human body in the thrombotic process with histological confirmation of microvascular occlusion and the presence of antiphospholipid antibodies (APA).
- Neonatal APS. This rare pathology occurs in newborns when thrombotic factors are transmitted transplacentally from mothers with high AFA. Often, pregnancy against the background of high APA titers is resolved by stillbirth of the fetus at an early stage of gestation.
- Serological variants of APS are seropositive and seronegative APS.
Currently, the diagnosis of “seronegative APS” is made with great caution, after excluding all other possible thrombophilias [2, 13].
The most common forms of APS in children are PAPS and VAPS.
J.-C. Pitte et al. (1993) developed a number of criteria to exclude PAPS:
- Facial erythema (butterfly).
- Discoid erythema.
- Ulceration of the mucous membrane of the mouth or pharynx.
- Arthritis.
- Pleurisy (without pulmonary embolism or left ventricular failure).
- Pericarditis (myocardial infarction or uremia is excluded).
- Persistent proteinuria more than 0.5 g/day (presence of immune complex glomerulonephritis).
- Lymphopenia less than 1000 cells in 1 μl.
- Antibodies to native DNA.
- Antibodies to extracted nuclear antigens.
- High titer antinuclear antibodies.
- Taking drugs that induce the production of antibodies to FL.
Antibodies to PL are presented in various classes (IgG, IgM, IgA) and have phospholipid specificity. PL is a heterogeneous group of molecules with different chemical structures and electrical charges. Negatively charged PLs include: phosphatidylglycerol - CL, phosphatidylinositol, and neutral ones - phosphatidylcholine and phosphatidylethanolamine. In the cell membrane, PL form a double layer with pronounced asymmetry. Negatively charged PL create a surface on which the assembly of enzyme complexes of the two main links of the coagulation cascade occurs. In one of them (the tenase complex), factor X is activated by a complex of factors IXa and VIIa, and in the other, the prothrombinase reaction, conversion of prothrombin to thrombin occurs by an enzyme complex consisting of factors Xa and Va (prothrombinase complex). The interaction of factors IXa, Xa and prothrombin with the lipid surface occurs through the formation of a calcium-dependent bridge between the gamma-carboxyglutamic acid residues of these proteins and the negatively charged polar groups of PL. Binding to the lipid surface leads to an increase in the local concentration and effective location of coagulation factors, which contributes to the maximum rate of the reaction. Any substances that interfere with the assembly of these complexes on the phospholipid surface, including antibodies to PL, can increase the level of thrombin formation and disrupt blood clotting. PL also includes VA, which in vitro prolongs various phospholipid-dependent coagulation tests [1, 10, 11].
Since the time of Hughes GRV, various theories have existed to explain the cellular and molecular mechanisms by which antiphospholipid antibodies to PL initiate thrombosis. One theory suggests activation of endothelial cells when an antibody attaches to PL, which is manifested by the expression of adhesion molecules, increased secretion of cytokines and prostacyclin metabolism.
The second theory is based on oxidant-dependent damage to the vascular endothelium. Oxidized low-density lipoprotein (LDL) is taken up by macrophages, leading to their activation, cytokine production, and subsequent endothelial cell damage. This is confirmed by the fact that antibodies to CL cross-react with oxidized LDL. Moreover, anti-PL antibodies bind to oxidized cardiolipin and likely recognize oxidized PS, phospholipid-binding proteins, or both.
A third theory suggests that anti-PL antibodies interact with phospholipid-binding proteins and/or modulate their coagulation regulating functions. This theory explains the molecular mechanisms of the influence of anti-FL antibodies on cofactor proteins: prothrombin, protein C, protein S, annexin V, prostacyclin and beta2-GP-I [4].
Clinical signs of APS are varied and depend on the location of non-inflammatory thrombotic vasculopathy of the vascular bed.
With venous thrombosis, damage to the deep veins of the lower extremities is noted. Some children may also develop pulmonary artery thrombosis, although this is very rare. Thrombosis of the venous vessels of the kidneys, liver, eyes and mesenteric vessels is characteristic.
Arterial thrombosis in children is observed in the cerebral arteries. The clinical manifestation of this pathology is a transient ischemic attack of the brain or a much less common stroke [2]. Thrombotic vasculopathy is characteristic of the arteries of the kidneys, liver and mesenteric vessels. The localization of arterial-venous thrombosis determines the variety of clinical manifestations of APS.
A high titer of antiphospholipid antibodies causes a wide range of pathologies of the central and peripheral nervous system: transient ischemic attack of the brain, ocular neuropathy, sudden hearing loss, partial seizures, episyndrome, chorea, transient general amnesia and psychosis.
Ravelli A., Martini A. (2007) in their studies detected a significant relationship between antibodies to CL of the IgM class and cerebrovascular manifestations in children with SLE. A similar study by doctors from Toronto showed that the pathogenetic factor in children with SLE are antibodies to beta2-GP-I [8]. The presence of high titers of antibodies to FL is typical for children with multifocal seizure syndrome [7].
The Pediatric Registry of Patients with APS (Ped-APS Registry) reported an association between hematological manifestations and VAPS in children. Of these, the most common are: thrombocytopenia, autoimmune hemolytic disease and bleeding. Clinical manifestations of damage to the cardiovascular system are variable and are characterized by damage to the heart valves, myocardium, arterial hypertension and intracardiac thrombi.
The skin manifestations of APS are varied and are manifested primarily by livedo reticularis - this is a vascular network in the form of bluish spots on the legs, feet, thighs, hands, which is especially noticeable during cooling. There may be a superficial rash in the form of pinpoint hemorrhages resembling vasculitis, or hemorrhage in the subungual bed, plantar and palmar erythema and skin nodules. The most severe cutaneous manifestations of APS are skin necrosis of the distal lower extremities and chronic leg ulcers.
According to the European Group for the Study of APS in Children (Euro-Phosphopolypid Project Group), of 1000 children with symptoms of APS, 53.1% were diagnosed with primary APS. The most characteristic manifestations of APS in children are venous thrombosis, thrombocytopenia, livedo reticularis. Neurological symptoms are observed in approximately 20% of patients [12].
According to our data, PAPS developed more often in boys with a burdened heredity (spontaneous abortions in the anamnesis of their mothers, stroke, hypertension, varicose veins, myocardial infarction). In clinical manifestations, symptoms of central nervous system damage predominated against the background of lower levels of anticardiolipin antibodies (ACA) and APA of the IgG class. VAPS was characterized by clinical signs of damage to the kidneys, lungs, skin and the presence of antibodies to DNA (Fig.).
It should be noted that an increase in the level of APA (usually transient), often detected against the background of a wide range of bacterial and viral infections, is rarely accompanied by the development of thrombotic complications. This is associated with differences in the immunological properties of APA in APS and infectious diseases: in patients with APS, beta2-GP-I-dependent anticardiolipin antibodies are formed. A number of viral infections (Epstein-Barr virus, herpes virus, etc.) contribute to an increase in the titer of antibodies to FL, therefore, for the reliability of the diagnosis of APS in children, observation and monitoring of clinical and immunological indicators of thrombosis over time is required [9, 13].
Management of patients with APS poses a serious challenge. APS is based on various pathogenetic mechanisms leading to thrombosis. The heterogeneity of clinical manifestations of APS, the lack of generally accepted clinical and laboratory parameters that allow predicting the recurrence of thrombotic manifestations, significantly complicates the choice of drug therapy for patients with APS. For example, the development of repeated thrombosis does not always correlate with changes in antibody titers to FL and the activity of the underlying disease in secondary APS.
Treatment of patients with APS is based on the prescription of indirect anticoagulants and antiplatelet agents (low doses of Aspirin), which are widely used for the prevention of thrombosis not associated with APS. In patients with VAPS, the underlying disease is also treated. Patients with risk factors for recurrent thrombosis over a long period should undergo intensive prophylaxis using low molecular weight heparin. It should be noted that for venous and arterial thrombosis, it is preferable to prescribe warfarin and acetylsalicylic acid in low doses, but the INR (international normalized ratio) should be maintained at a level of 2–3 (for venous thrombosis) and > 3 (for arterial thrombosis). The inclusion of acetylsalicylic acid in the complex therapy of APS leads to normalization of blood coagulation and an increase in the number of platelets in peripheral blood [4, 6]. Individual clinical observations or small open trials report the effectiveness of plasmapheresis, intravenous immunoglobulin, prostacyclin, fibrinolytic drugs, and fish oil preparations in women with obstetric pathology. Treatment with plasmapheresis, high doses of glucocorticoids (including pulse therapy) and cytostatics is used in the treatment of “catastrophic” APS [4, 15].
The study of the development mechanisms, clinical features and approaches to treatment of APS continues to be one of the most pressing multidisciplinary problems of modern medicine, the solution of which requires the combined efforts of specialists from various fields of medicine - pediatricians, rheumatologists, cardiologists, neurologists, obstetricians-gynecologists, immunologists.
Literature
- Barkagan Z. S., Momot A. P. Diagnosis and controlled therapy of hemostasis disorders. Ed. 2nd, supplemented. M.: Newdiamed, 2001.
- Kalashnikova L. A. Neurology of antiphospholipid syndrome. M.: Medicine, 2003, p. 16–20
- Kalinina N. M., Drygina L. B., Sokolyan N. A. Autoimmune pathology of the endothelium // Medical immunology. 2004. T. 6. No. 1, 1–15.
- Nasonov E. L. Antiphospholipid syndrome. M.: Litterra, 2004, p. 337–343.
- Nasonov E. L. Rheumatology // Clinical recommendations. M., GEOTAR-Media, 2008, p. 145–146.
- Reshetnyak T. M. Antiphospholipid syndrome. Low molecular weight heparins in the treatment of antiphospholipid syndrome and new prospects. CONSILIUM-MEDICUM: Volume 08/N 2/2006.
- Asherson RA Primary antiphospholipid syndrome // J. Rheumatol. 1988. V. 15. P. 1742–1746.
- Asherson RA Harris EN Anticardiolipin antibodies-clinical association // Postgrad. Med. J. 1986. Vol. 62. P. 1081–1087.
- Avcin T., Cimaz R., Silverman ED, Cervera R.., Gattorno M., Garay S., Berkun Y., Sztajnbok FR, Silva CA, Campos LM, Saad-Magalhaes C., Rigante D., Ravelli A. , Martini A., Rozman B., Meroni PL Pediatric antiphospholipid syndrome: clinical and immunological features of 121 patients in an international registry//Pediatrics Nov. 2008; 122(5):1100–1107.
- Hanly JG Antiphospholipid syndrome // CMAJ. 2003, June 24; 168(13):1675–1682.
- Heshmat NM, El-Kerdany TH Serum levels of vascular endothelial growth factor in children and adolescents with systemic lupus erythematosus // Pediatr Allergy Immunol. Jun 2007; 18 (4): 346–353.
- Piette J.-C., Cervera R., Font J. et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1000 patients // Arthritis Rheum. 2002. 46: 1019–1027.
- Ravelli A., Martini A. Antiphospholipid syndrome in pediatrics //Rheum Dis Clin North Am. Aug 2007; 3 (3): 499–523.
- Wilson WA, Gharavi AE, Koike T. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome // Arthritis Rheum. 1999. 42: 1309–1311.
- Xiao HJ, Yang JY, Gao TJ, Huang JP, Yao Y., Zhang Y. Clinical significance of antiphospholipid antibody in pediatric patients and review of literature // Zhonghua Er Ke Za Zhi. Aug 2004; 42(8):571–573.s
G. A. Novik , Doctor of Medical Sciences, Professor N. M. Kalinina , Doctor of Medical Sciences, Professor L. N. Abbakumova , Candidate of Medical Sciences, Associate Professor K. G. Kiknadze State Pedagogical Academy , St. Petersburg
Buy an issue with this article in pdf
Forecast
In the case of functional noise, the prognosis is always favorable. This is not a disease or a manifestation of a pathological process. There are no disturbances in the activity of cardiac structures or hemodynamic problems.
Outside of organic disorders, anatomical defects are also positive. It is possible to achieve complete recovery in 90-95% of cases.
Compensated defects (not all) can be cured in 80% of situations by surgical methods.
In the most severe cases, the survival rate is 30-40%, which is still quite high. The main thing that parents need to remember is that they should closely monitor the child’s well-being and, if necessary, urgently contact a cardiologist for a full diagnosis.
What is spinal muscular atrophy and its types
This term combines several different types of hereditary diseases accompanied by limited motor abilities. This explains the fact that in some cases, disorders are detected not in infancy, but in adolescents or already mature people.
The disease was first described in 1891 by G. Werdnig and in 1892 it was identified as a separate nosological unit by J. Hoffman, thanks to whose efforts it received its second name. About half a century later, E. Kugelberg and L. Welander discovered another similar disease, developing at a later age and characterized by a more favorable course.
The following forms of pathology are distinguished:
- SMA 0;
- SMA 1 (severe form);
- SMA 2 (intermediate form);
- SMA 3 (mild form);
- SMA 4 (late form).
Cause of spinal muscular atrophy in children
What they all have in common is that the cause of their occurrence lies in the mutation of the recessive gene of chromosome 5 SMN. This leads to disruptions in the production of proteins in the body, which are the building blocks of all cells. As a result, the motor neurons of the spinal cord suffer and are gradually destroyed. Since without them it is impossible to transmit nerve impulses to muscle fibers, they gradually atrophy, which causes loss of the ability to move.
Fortunately, even if both parents have the SMN gene mutation, they have a 75% chance of having a healthy child. But almost always he will also be a carrier of this gene. Therefore, when planning a pregnancy, it is worth undergoing genetic testing, especially if there are cases of SMA in the family.
SMA 0
This is a congenital disease, the signs of which are usually detected in the maternity hospital. It is rare and is often combined with SMA-1. This species is characterized by an absolute lack of mobility, muscle weakness, lack of tendon reflexes and limited functionality of the knee joints. From the first days of life, the child suffers from breathing problems.
It is important to differentiate spinal muscular atrophy from perinatal encephalopathy and birth injuries, but if with them the condition of children gradually improves, then with SMA it does not change. Moreover, complications are often added, which almost always lead to the death of infants during the first month of life.
SMA 1 or Werding-Hoffman disease
This type of spinal muscular atrophy is characterized by a very severe course. It is usually detected before 6 months of age and is accompanied by muscle weakness and periodic spasms, which is difficult to notice due to the anatomy of children in the first year of life (the presence of pronounced subcutaneous fat).
The disease also manifests itself regularly as tremors running through the tongue, decreased gag, sucking, and swallowing reflexes. This leads to serious feeding difficulties. There is a violation of salivation and cough. The child often screams loudly.
Since the chest muscles are not developed enough, you may notice that the shape of the chest is flatter. In addition, children with this pathology lie and sleep in the “frog” position: with their shoulders and hips pulled to the sides and their limbs bent at the knees and elbows. They are able to learn to hold their head up by six months (it is often smaller than that of healthy children), but they are not able to sit independently or take an upright body position.
This form of spinal muscular atrophy may be accompanied by mental retardation and congenital heart defects. Children are susceptible to severe breathing problems and the development of pneumonia. In this regard, more than half of children do not live to see 2 years of age and only 10% can celebrate their 5th birthday. The cause of death is pneumonia, cardiac arrest or respiratory failure.
SMA 2 or Dubowitz disease
The disease is detected in children from 6 months to 1.5–2 years. Therefore, this form of SMA is often called late infantile. Typical for her:
- weakness and tremors in the muscles;
- tremor of fingers, tongue;
- stiffness of movement due to limited mobility of the limbs;
- developmental delay;
- underweight
Children with this diagnosis are able to sit, play, and eat independently, but cannot stand or move around. Unfortunately, the pathology tends to progress, which leads to a gradual weakening of the muscles of the chest and neck, resulting in the inability to hold the head straight and often it hangs limply. Then tendon reflexes disappear, the voice weakens and swallowing disturbances are noted.
Life expectancy with this diagnosis is about 10–12 years. But a third of patients die before the age of 4 years.
SMA 3 or Kugelberg-Welander disease
Spinal muscular atrophy of this type is usually diagnosed after 2 years. It is also manifested by muscle weakness, but not to the same extent as in SMA 1 or even SMA 2. Patients can stand independently, but only for a short period of time. Due to muscle atrophy, this is difficult for them.
Despite the existing disease, the child develops normally until the age of 10–12, which can mislead his family and raise doubts about the correctness of the diagnosis. But, reaching this time point, the first signs of SMA appear. The child begins to stumble more often than usual, falls and is unable to perform physical work or play sports, and often experiences fractures. Gradually, running and then walking become more and more difficult due to limited joint mobility. Subsequently, the teenager loses the ability to move without a wheelchair.
The progression of the pathology leads to the occurrence of severe scoliosis, which entails a change in the shape of the chest and the appearance of breathing difficulties. This is where the main threat of the disease to life lies.
SMA 4
This type of disease includes several different amyotrophies that do not affect life expectancy, but lead to disability:
- bulbospinal Kennedy;
- distal Duchenne-Aran;
- peroneal Vulpiana.
What they have in common is that the first clinical signs of the disease appear between 16 and 60 years of age, most often at 35–40 years of age. This is accompanied by extinction of tendon reflexes and noticeable muscle spasms. With Duchenne-Harand atrophy, the hands are most affected, and Vulpian disease is characterized by a change in the shape of the shoulder blades to pterygoid.