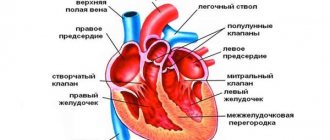
Levels of the circulatory system
Conventionally, all blood supply to organs and systems of the body can be divided into three levels:
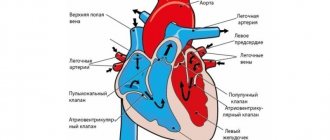
- Systemic circulation is formed by large vessels that transport blood throughout the body.
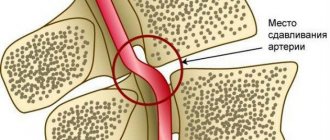
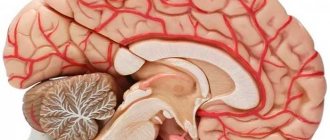
- Organ blood circulation is formed by medium-diameter vessels that provide blood supply to individual organs depending on their need for oxygen. For example, the brain is supplied with blood very abundantly, since it needs a lot of energy, and therefore oxygen.
- Microcirculation - includes the smallest vessels that are in direct contact with cells and tissues.
Typical lymphodynamic disorders
1. Relevance of the topic: pathological processes occurring in the capillary-trophic region are the leading mechanism underlying damage to various organs and systems.
The trophic function of capillary-connective tissue structures and their close relationship with lymphatic drainage structures are extremely vulnerable and are very often damaged by various factors of both external and internal structure. Blood circulation in the area of the peripheral vascular bed, in addition to blood movement, ensures the exchange of water, electrolytes, gases, essential nutrients and metabolites through the blood-tissue-blood system. In an organ or tissue, in response to functional and structural changes in them, local circulatory disorders may occur: arterial and venous hyperemia, ischemia, stasis. Thrombosis and embolism can lead to local circulatory disorders.
https://www.youtube.com/watch?v=https:accounts.google.comServiceLogin
Lymphatic circulation insufficiency is a condition in which the lymphatic vessels do not perform their main function - providing constant and effective drainage of the interstitium.
Microcirculation: what is it?
Microcirculation is the movement of blood through the microscopic, that is, the smallest, part of the vascular bed. There are five types of vessels that are part of it:
- arterioles;
- precapillaries;
- capillaries;
- postcapillaries;
- venules.
What’s interesting is that not all vessels in this channel function simultaneously. While some of them are actively working (open capillaries), others are in “sleep mode” (closed capillaries).
Regulation of blood movement through the smallest blood vessels is carried out by contraction of the muscular wall of arteries and arterioles, as well as by the work of special sphincters, which are located in the post-capillaries.
Problems with microcirculation
Disruption of the capillary bed underlies most pathological processes. At the microscopic level, arterioles spasm or are blocked by microthrombi from blood cells. This leads to a lack of oxygen and a transition of cells to the anaerobic (without the participation of oxygen) process of glucose breakdown.
As a result, acidic metabolic products accumulate in the body, in particular lactic acid or lactate, which greatly aggravates metabolic disorders.
Some diseases whose pathogenesis is based on microcirculatory disorders:
- Diabetes. One of the main complications is microangiopathy, that is, pathology of the capillary bed. Poor glycemic control leads to thickening of capillary walls and impaired transport across membranes. Tissue nutrition is disrupted, trophic ulcers appear on the legs. The lesion affects almost all vessels, even the retinal arterioles in the eyes.
- Coronary heart disease (CHD). The main cause of IHD is the deposition of cholesterol on the walls of blood vessels, the formation of atherosclerotic plaques. These factors disrupt normal peripheral blood flow, and the vessels become rigid. Not only the myocardium suffers, but also other organs. Trophic disorders in the lower extremities are often caused by obliterating atherosclerosis of blood vessels.
- Stroke or cerebrovascular accident. Thrombosis or rupture of a cerebral vessel leads to ischemic or hemorrhagic stroke, respectively. Damage to nerve cells (neurons) occurs due to blockage of the smallest vascular bed.
- Kidney diseases. Renal pathology is associated with impaired elimination of fluid and nitrogen metabolism products. The gradual accumulation of urea also negatively affects vascular perfusion, disrupting normal tissue trophism.
Not all pathological processes whose pathogenesis is based on microcirculatory disorders are listed here. The presence of systemic atherosclerosis always aggravates the situation. For patients with a large number of cholesterol plaques and thickening of the vascular walls, for example, it is much more difficult to recover from a stroke.
to the point ↑
Structural features
The microvasculature has a different structure, depending on the organ in which it is located.
For example, in the kidneys, the capillaries are collected into a glomerulus, which is formed from the afferent artery, and from the glomerulus of capillaries itself the efferent artery is formed. Moreover, the diameter of the incoming one is twice as large as the outgoing one. This structure is necessary for the filtration of blood and the formation of primary urine.
And in the liver there are wide capillaries called sinusoids. Both oxygenated arterial and oxygen-poor venous blood enter these vessels from the portal vein. Special sinusoids are also present in the bone marrow.
Pathological conditions
Blood flow in the microvasculature depends on the constancy of the internal environment of the body. The work of the heart and endocrine glands has the greatest influence on the normal function of blood vessels. However, other internal organs also have an influence. Therefore, the state of microcirculation reflects the functioning of the body as a whole.
Conventionally, all pathological conditions of microvasculature vessels can be divided into three groups:
- changes inside the vessel - disruption of blood flow inside it with an increase in its viscosity and disruption of the stability of blood cells;
- violation of the integrity of the vessel wall - increased permeability of the vascular wall;
- changes outside the vessel - endocrinological diseases, cardiac dysfunction.
Anatomy and physiology of the microvasculature
The term microcirculation in the broad sense of the word understands not only blood flow and lymph flow in microvessels, but also metabolic processes occurring through the wall of microvessels, as well as interstitial (extravascular) transport of fluid and the substances, cells and various structures it contains.
Microvessels include:
- vessels of the arteriolar type - arterioles, metarterioles, precapillaries and capillaries with a diameter not exceeding 100 microns;
- vessels of the venular type - postcapillaries, venules, the diameter of which does not exceed 200 microns;
- lymphatic microvessels - lymphatic capillaries, postcapillaries and microvessels with a diameter of no more than 300 microns;
- anastomoses (shunts) connecting two arterioles (arteriolo-arteriolar), two venules (venulo-venular), an arteriole with a venule (arteriolo-venular).
The main difference between microvessels and macrovessels is that, in addition to transport, they perform an exchange function. It should be noted that metabolism occurs through the wall of all microvessels - from arteriole to venule - and not only through the wall of capillaries, as previously assumed. A feature of lymphatic macro- and microvessels is a constant two-way exchange from tissue to vessel and from vessel to tissue throughout the entire length until the final section of the thoracic lymphatic duct flows into the right venous angle and into the right atrium. In this regard, the composition of lymph in each section of the lymphangion (the distance between adjacent valves of the lymphatic vessel) is different. The increased permeability of lymphatic microvessels is explained by the thin structure of their wall, which does not have a continuous basement membrane, and in lymphatic capillaries and post-capillaries there is no basement membrane. Their wall consists of a single row of imbricated endothelial cells. Interendothelial channels freely pass not only individual cells, but also entire conglomerates, which eliminates the need for lymphovenous anastomoses.
One of the important structures of the microvasculature is the precapillary sphincter (Fig. 1), which is a section of the precapillary containing two smooth muscle cells located at the beginning of the precapillary. Only one red blood cell can pass through it without deformation. In narrower capillaries with a diameter of about 5 microns, the red blood cell is necessarily deformed, stretching 3-7 times in length (depending on the speed of blood flow and the pressure gradient in the capillary). The shape of the red blood cell can be used to judge the speed of blood flow in the microvessel. At high speeds, the cells are elongated; at low speeds, the red blood cells take on a more rounded shape (Fig. 2).
The reason why we dwell in detail on the structure of the precapillary sphincter is explained by the importance of its role in the development of pathology. Smooth muscle cells of the precapillary sphincter are hypersensitive to catecholamines (adrenaline, norepinephrine). For example, the sensitivity of a precapillary sphincter smooth muscle cell to adrenaline is 100 times greater compared to a similar arteriole cell with a diameter of 50 μm and 50 times greater compared to a similar arteriole cell with a diameter of 20 μm (see Fig. 1).
This feature of the smooth muscle cells of the precapillary sphincter is of particular importance. Under conditions of acute stress, the release of a significant amount of catecholamines is accompanied by spasm of the coronary and other vessels. Acute pain in the heart area signals the need to take a vasodilator such as nitroglycerin. Everyone knows this: both the patient and the doctor.
A completely different situation develops under conditions of chronic stress, by which we mean conditions accompanied by a person’s long-term dissatisfaction with the reality around him: long-term worries due to the loss of loved ones or loss of a job; conflicts in the family or work team, constant lack of time, insufficient sleep, lack of positive emotions and much more that causes irritation in a person. In these situations, the release of catecholamines will be insignificant: there will be no pain attack, disturbances in central hemodynamics, blood pressure will remain within normal limits. However, the hypersensitive cells of the precapillary sphincter will respond by contracting even to minute doses of catecholamines. Its lumen will decrease, and not a single red blood cell will be able to pass through the precapillary sphincter. The tissues that are supplied with blood through capillary networks will continue to receive plasma flow, but will not receive the oxygen carried by red blood cells. As a consequence of prolonged narrowing of the precapillary sphincter in response to small doses of catecholamines in the blood under conditions of chronic stress, chronic hypoxia of tissues and organs supplied by the capillary networks will occur.
Figure 2. Changes in the shape of an erythrocyte at different blood flow rates in the microvessels of the mesentery of the small intestine of a rat. Elongated red blood cells in the capillary (3) and arteriole (5) at normal blood flow speed; oval shape - with sharply slowed blood flow (4); “square” red blood cells when blood flow slows down (1, 2). Biomicroscopy. Magnification: vol. x 70, approx. x 3.
The future scenario is far from the most optimistic: from a decrease in the functional activity of organs to the development of various diseases, such as cancer. Under hypoxic conditions, cells degrade faster; cells of the immune system perceive them as foreign and phagocytose. The rapid death of cells that have not reached maturity and the increased growth of new ones, which are also soon destroyed, will lead to malignancy of cells and a tumor process.
Intravascular changes
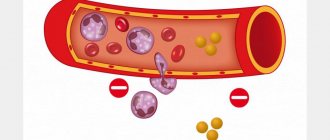
Slowing of blood flow in the vessels, which can manifest itself both in specific diseases, thrombocytopathies (impaired platelet function) and coagulopathies (blood clotting disorders), and in pathologies that can occur in various diseases of the body. These conditions include red blood cell aggregation and sludge syndrome. In fact, these two processes are successive stages of one phenomenon.
First, temporary attachment of red blood cells occurs using surface contacts in the form of a column (erythrocyte aggregation). This condition is reversible and usually short-term. However, its progression can lead to strong gluing (adhesion) of blood cells, which is already irreversible.
This pathology is called the sludge phenomenon. This leads to a slowdown and complete cessation of blood flow in the vessel. Venules and capillaries are usually clogged. The exchange of oxygen and nutrients stops, which further causes ischemia and tissue necrosis.
Transmural microcirculation disorders
The liquid part of the blood and lymph circulates through the wall of the microvessel (characterized by the concept of “vascular wall permeability” for plasma) and the formed elements of blood (described by the concept of “emigration”).
In accordance with the predominance of permeability or emigration disorders, transmural disorders of microcirculation of blood and lymph components are divided into two categories:
Ú disorders of the permeability of microvascular walls for fluid;
Ú violation of the emigration of blood cells through the wall of microvessels.
Violation of the permeability of the microvessel wall for liquid
Under various pathological conditions, the volume of movement of blood plasma and/or lymph through the vessel wall can either increase or decrease excessively (inadequately).
Increased movement of fluid through the wall of microvasculature vessels
The main reasons for the increase in the permeability of microvascular walls are shown in Fig. 23.54.
Y LAYOUT Insert file “PF_Fig.23.54” MS Y
Rice. 23.54. The main reasons for the increase in the permeability of microvascular walls for fluid.
The main consequence of increasing the permeability of microvascular walls is the potentiation of fluid circulation mechanisms:
Ú filtration (transport of liquid along a hydrostatic pressure gradient);
Ú transcytosis (energy-dependent pinocytosis);
Ú diffusion (transfer of liquid without energy consumption);
Ú osmosis (directed diffusion of liquid along an osmotic pressure gradient).
Reduced permeability of the walls of microvasculature vessels
Reasons for decreased permeability of microvascular walls:
Ú thickening of the walls of microvessels (for example, in chronic vasculitis) ;
Ú compaction of the walls of microvessels (for example, due to their calcification) .
The most significant consequence of a decrease in the permeability of microvascular walls is a decrease in the efficiency of the mechanisms for moving fluid through them:
Ú filtration;
Ú diffusion;
Ú transcytosis;
Ú osmosis.
Impaired movement of blood cells through the wall of a microvessel
The emigration of leukocytes through the wall of microvessels (into the tissue and back) normally occurs continuously. Leukocytes perform in tissues the function of immunobiological surveillance of the individual and constant antigenic composition of the body. Red blood cells and platelets do not have this ability.
Under various pathological conditions, the emigration of leukocytes through the wall of a microvessel, as a rule, increases; red blood cells and platelets move into the tissue passively with the flow of blood plasma.
Typical forms of pathology of the movement of blood cells through the wall of a microvessel into the tissue include:
Ú excessive release of erythrocytes from microvessels with the development of microhemorrhages (for example, with vasculitis or hemorrhagic syndromes);
Ú excessive movement of platelets into the tissue (usually together with red blood cells).
Extravascular microcirculation disorders
Extravascular (extravascular) microcirculation disorders are accompanied by an increase or decrease in the volume of intercellular fluid, which leads to a slowdown in its outflow into the vessels of the microvasculature.
The reason for the excessive slowdown in the outflow of interstitial fluid and the excessive increase in its volume is local tissue pathological processes. Most often this is:
Ú inflammation;
Ú allergic reactions;
Ú growth of neoplasms;
Ú tissue sclerosis;
Ú venous hyperemia;
Ú stasis.
Consequences of slowing the outflow of interstitial fluid and excessive increase in its volume:
Ú increase in the content of products of normal and disturbed metabolism in the interstitial fluid (a number of such metabolites can have cytotoxic and cytolytic effects);
Ú ion imbalance (this promotes tissue edema, disrupts the formation of MP and AP);
Ú formation of excess and/or activation of biologically active substances (for example, TNF-a, procoagulants, membrane attack complex), which can aggravate cell damage, potentiate disorders of blood and lymph circulation, and plastic processes;
Ú disturbance of the exchange of O2, CO2, substrates and metabolic products;
Ú compression of cells by excess interstitial fluid.
Reasons for excessive slowing of the outflow of interstitial fluid, combined with a decrease in its outflow from the interstitial space:
Ú hypohydration of the body, tissues and organs (for example, as a result of prolonged diarrhea, plasmorrhagia, with intense sweating);
Ú decreased lymph formation (for example, with tissue ischemia or systemic hypovolemia);
Ú a decrease in the efficiency of fluid filtration in arterioles and precapillaries and/or an increase in its reabsorption in postcapillaries and venules (for example, during dystrophic and sclerotic processes in tissues).
The consequences of an excessive decrease in the outflow of interstitial fluid are similar to those observed with an increase in the volume of interstitial fluid, combined with a slowdown in its outflow (see above).
Capillary-trophic insufficiency
Capillary-trophic insufficiency is a condition characterized by the following disorders leading to metabolic disorders and plastic processes in tissues and organs:
Ú blood and lymph flow in the vessels of the microvasculature;
Ú transport of fluid and/or blood cells through the walls of microvessels;
Ú outflow of intercellular fluid (Fig. 23.55).
Y LAYOUT Insert file “PF_Fig.23.55” MS Y
Rice. 23.55. Signs of capillary-trophic insufficiency.
Consequences of capillary-trophic insufficiency
As a result of the disorders of microcirculation of blood, lymph and intercellular fluid described above in tissues and organs, the following develops:
Ú various forms of dystrophy;
Ú disorders of plastic processes (recovery of damaged cells and synthesis of new cells and extracellular structures;
Ú dysfunction of tissues, organs and the body as a whole.
Sludge
Sludge is a typical form of pathology of the aggregate state of blood.
Sludge is characterized by adhesion, aggregation and agglutination of blood cells, causing its separation into plasma and dense conglomerates of erythrocytes, leukocytes, platelets, as well as the formation of blood clots.
Conglomerates and blood clots slow down blood flow, obstruct microvasculature vessels and lead to microcirculation disorders.
Causes of sludge:
Ú disturbances of central hemodynamics (for example, with heart failure, venous stagnation, ischemia, pathological forms of arterial hyperemia);
Ú increased blood viscosity (for example, in conditions of hemoconcentration, hyperproteinemia, polycythemia);
Ú damage to the walls of microvessels (during local pathological processes: inflammation, immunopathological conditions, tumor growth, etc.).
Pathogenesis of sludge
The main links in the pathogenesis of sludge are presented in Fig. 23.56.
Y LAYOUT Insert file “PF_Fig.23.56” MS Y
Rice. 23.56. The main links in the pathogenesis of sludge. FEC - formed elements of blood.
Consequences of sludge
Sludge causes significant disturbances in microcirculation and metabolism in tissues and organs, which lead to:
Ú intravascular microcirculation disorders (slowdown, up to stasis; turbulent blood flow; opening of arteriovenular shunts);
Ú violations of the transmural movement of blood cells, as well as its outflow from tissues;
Ú metabolic disorders in tissues and organs with the development of dystrophies and dysplasias.
In general, the combination of the above changes leads to the development of capillary-trophic insufficiency.
This leads to an important conclusion that the phenomenon of sludge can be:
Ú as a cause of microcirculation disorders (in cases where it develops primarily);
Ú and as a consequence of intravascular microcirculation disorders (during their primary development).
Chapter 24
Typical forms of pathology of the external respiratory system
Respiration - the exchange of oxygen and carbon dioxide - occurs through their diffusion. The determining factor in this process is the difference in the partial pressure of the gases.
Conventionally, “internal” (tissue) and “external” (alveolar) respiration are distinguished.
Tissue respiration is the bidirectional diffusion of gases between the lumen of blood capillaries and cells (the term “tissue respiration” also has a broader meaning: utilization of O2 in the process of cell metabolism).
External respiration is the bidirectional diffusion of gases between the alveoli of the lungs and the blood of the capillaries of the interalveolar septa through the aerohematic barrier.
The external respiration apparatus includes:
Ú respiratory tract;
Ú respiratory department of the lungs;
Ú chest (including its osteochondral frame and neuromuscular system);
Ú nerve centers for regulating breathing.
The external respiration apparatus provides:
Ú alveolar ventilation (bidirectional diffusion of oxygen and carbon dioxide through the alveolar capillary membrane);
Ú perfusion of lung tissue (blood supply).
Partial or combined disorders of the functioning of the external respiratory apparatus can lead to respiratory failure.
Respiratory failure is a condition characterized by the development of respiratory hypoxia and, as a rule, hypercapnia as a result of impaired gas exchange function of the lungs.
Assessment of respiratory function
To characterize the function of external respiration, indicators are used that allow one to assess the function of the airways and respiratory part of the lungs (additional information about indicators of external respiration function is given in the articles “Capacity”, “Volume” and “Speed” of the Appendix “Reference of Terms”).
When studying lung function, they analyze mainly the state of indicators characterizing:
Ú pulmonary volumes,
Ú volumetric expiratory flow rate,
Ú diffusion capacity of the alveolocapillary membrane.
Lung volumes are characterized by static and dynamic indicators:
Ú indicators of static pulmonary volumes reflect the state of the elastic properties of lung and chest tissue;
Ú indicators of dynamic pulmonary volumes characterize the patency of the airways.
Expiratory volumetric flow rate is the maximum speed of air flow in the respiratory tract during forced exhalation. The speed of the air flow depends on the state of the lung volumes and the force of exhalation. The air flow increases with increasing force of exhalation, especially at its beginning (this flow can account for more than 75% of the vital capacity of the lungs during the first second of exhalation). The volumetric flow rate of exhalation is also influenced by the elastic traction of the lung tissue and the resistance of the airways to air flow.
Diffusion capacity, or diffusion capacity (Dc) of the airborne barrier is an indicator of the efficiency of transport of a certain gas from the alveoli into the blood of the capillaries of the lungs.
Spirometry (spirography) and its indicators
Spirometry (spirography) is the measurement of vital capacity and other lung volumes using a spirograph (a device for continuous graphical recording of changes in the volumes of inhaled and exhaled air).
The spirogram is recorded at the moment of maximum deep inspiration and calm exhalation. After this, inhalation and exhalation are recorded again, but with maximum effort.
Spirometry indicators make it possible to differentiate obstructive and restrictive disorders of alveolar ventilation, assess the degree of respiratory failure and its dynamics during treatment.
Many spirogram indicators are expressed in relative values (usually in %) from their average values in the population (gender, age, height are taken into account). Fluctuations in the indicator in the range from 80 to 120% are considered normal.
· Tidal volume (VT) - the volume of air entering the lungs in one breath during quiet breathing (the norm is 500–800 ml). The part of the DO involved in gas exchange is called the alveolar volume (AO); the remainder - about 30% of the total volume - is anatomically dead space, or harmful volume .
· VC - the maximum volume of air expelled from the lungs following maximum inhalation. VC progressively decreases in restrictive lung diseases. In this regard, vital capacity, in combination with the diffusion capacity of the lungs, helps to assess the course of the disease and the effectiveness of its treatment in patients with restrictive pathology.
· Forced vital capacity (FVC) is assessed similarly to VC, except that breathing is performed with the maximum possible force and speed. Forced exhalation is accompanied by a narrowing of the airways, slowing down its speed.
· Forced expiratory volume in 1 second (FEV1) - the volume of air expelled with maximum effort from the lungs during the first second of exhalation after a deep inhalation, i.e. this is the part of the FVC exhaled in the first second. FEV1 reflects the condition of the large airways and is often expressed as a percentage of VC (normal FEV1 = 75% VC).
· FEV1/FVC - the ratio of FEV1 to FVC (Tiffno index), expressed as a percentage (normally greater than or equal to 70%). The FEV1/FVC value is directly proportional to the expiratory force. This indicator is important for identifying obstructive breathing disorders, as well as for diagnosing restrictive respiratory disorders. A decrease in FEV1 only (FEV1/FVC < 70%) indicates obstruction; a decrease in both indicators (FEV1/FVC ³70%) indicates a restrictive pathology.
· Average expiratory volumetric flow rate (SOS25%–75%) - the forced expiratory flow rate in its middle (i.e. between 25% and 75% FVC); otherwise it is referred to as the maximum mid-expiratory flow . SOS25%–75% reflects the condition of the small airways. It is more informative than FEV1 in identifying early obstructive disorders.
· Peak volumetric expiratory flow rate (expiratory power) - the maximum volumetric flow rate that the subject can develop during forced exhalation. This is an indicator of airway patency at the level of the trachea and large bronchi. Depends on the patient's muscle effort.
Other lung volumes
Obtaining other indicators of lung volumes requires the use of not only spirometry, but also a test with helium dilution in the lung air (which allows you to determine the volume of gas in the lungs).
· Total lung capacity (TLC) - the volume of air contained in the lungs at the height of maximum inspiration.
· Functional residual capacity (FORC) - the volume of air remaining in the lungs at the end of normal exhalation. Reflects the state of rest of the lungs and chest wall (the volume of the lungs in conditions of equilibrium of the elastic traction of the lungs directed inward and the traction of the chest directed outward. FOY is represented by two components:
Ú p expiratory reserve volume (ERV) - part of the EFV, which can be expelled from the lungs during maximal exhalation;
Ú about stagnant lung volume (SLV) - the volume of air remaining in the lungs after the most intense exhalation (normally 25–30% of SVV).
· Lung volume ratios:
OEL = ZHEL + OOL
OOL = FOYO – ROvyd
Study of other lung functions
· The diffusion capacity (diffusion capacity, Dc) of the lungs for carbon monoxide (DcCO) reflects the state of the alveolar-capillary membrane: the air-hematic barrier. DsCO is determined by measuring the amount of carbon monoxide (CO) released from the alveolar air into the blood of the pulmonary capillaries after the patient has inhaled a known amount of CO (0.1%). Express DsCO in ml/min/mmHg.
· Lung compliance (extensibility) . The elasticity of the lungs determines the ratio of changes in lung volumes and transpulmonary pressure (the difference in pressure in the alveoli and in the pleural cavity). To stretch the lung to a given volume, a certain force is required, which consists of the elastic traction of the lung, directed inward, and the elastic traction of the chest wall, directed outward. Normally, at rest at the end of expiration (i.e. in the FOY position), the elastic traction of the lung is completely balanced by the elastic traction of the chest wall. With a full inhalation (i.e., with OIL), the lungs reach their maximum elastic traction. With full exhalation (i.e., with expiratory reserve volume), the chest wall reaches its maximum elastic traction. The dependence of transpulmonary pressure on lung volume is displayed in the form of a lung compliance curve. Compliance , or compliance ( C ), is determined by the slope of the pressure-volume curve ( P - V ) above the level of tidal volume:
C = V/P (normal 200 ml/cm H20)
Loss of elastic traction of the lung (eg,
with emphysema) increases compliance, shifting the compliance curve to the left.
An increase in elastic recoil of the lung (eg ,
in restrictive lung diseases such as idiopathic pulmonary fibrosis) decreases compliance, shifting the compliance curve downward and to the right.
· Airway resistance (ARR). This indicator reflects the condition of the large airways, since they create 80–90% of the resistance to air flow. TDP is usually determined by dynamic lung volumes and expiratory volumetric flow rates. SDP values are increased in obstructive pulmonary diseases and decreased in restrictive pulmonary diseases.
Spirometric signs of respiratory failure
Obstructive alveolar ventilation disorders
Volumetric speeds . With alveolar hypoventilation due to airway obstruction, the FEV1/FVC indicator decreases (less than 70%). This indicator is closely related to exhalation time. However, even with significant obstruction of the peripheral airways, FEV1/FVC may be within the average physiological norm. In such a situation, airway obstruction can be identified by a decrease in SOS of 25%–75% (up to 60% or lower of the expected value).
Lung volumes . Changes in tidal lung volumes can occur with moderate to severe airway obstruction. Measuring lung volumes helps to recognize overdistension of the lungs due to premature closure of the airways (expiratory bronchial collapse).
It is known that during forced expiration, the distal parts of the respiratory tract close before air is expelled, which leads to overdistension of the lungs, causing an increase in FLC, TLC and TLC/VC.
When the patency of the small airways is impaired, “air traps” are formed in them, increasing the FEV, while the FER and FEV1 remain normal.
With pulmonary emphysema, the destruction of the walls of the alveoli and the loss of elastic traction by the lungs cause an increase in TLC.
Restrictive alveolar ventilation disorders
Volumetric speeds . FEV1/FVC and SOS25–75% in the restrictive form of pulmonary hypoventilation may be normal or increased due to increased draft of the airway walls.
Lung volumes
The most informative sign of restrictive pulmonary ventilation disorders is a decrease in VC and TLC.
Rigidity of the lungs with their restrictive lesions increases the elastic traction of the lungs and thereby reduces the FER.
Rigidity of the chest wall (for example, with kyphoscoliosis) reduces lung volumes because it limits the expansion of the lungs.
The extensibility of the lungs is also limited due to an increase in their elastic traction.
Airway resistance (ARR) to air flow is reduced, since, thanks to elastic forces, the airways are expanded at the level of any lung volume.
Arterial blood gases
The partial pressure of oxygen ( pO2 ) and carbon dioxide ( pCO2 ), as well as pH , are important parameters for assessing lung function. They indicate the state of gas exchange between the lungs and blood.
pO2 . In the absence of pathology, pO2 decreases with age due to the loss of elasticity of the lungs (normally it is 90 mm Hg at the age of 20 and about 70 mm Hg by the age of 70).
A decrease in pO2 below normal indicates hypoxemia, but tissue oxygen saturation does not decrease significantly until pO2 falls below 60 mm Hg.
pCO2 . Reflects the state of alveolar ventilation (normally 35–45 mm Hg). Hypercapnia (respiratory acidosis, high pCO2) indicates hypoventilation.
pH . Comparison of arterial pH (normally 7.35–7.45) with pCO2 makes it possible to distinguish respiratory disorders of OS from metabolic ones. For example ,
if paCO2 and pH are inversely proportional (one indicator decreases while the other increases), the CBS disorder is of a respiratory nature.
Violations of the ventilation-perfusion (V/Q) ratio
Optimal ventilation and pulmonary blood flow ensure sufficient oxygen delivery to tissues and adequate elimination of carbon dioxide from the body.
On average, the V/Q is 0.8 (normally, a physiological imbalance of V/Q , caused by the “discharge” in the lungs of approximately 2% of the arterial blood volume into the venous blood through arteriovenous shunts without gas exchange).
Previous24Next
Destruction of the vascular wall
Violation of the integrity of the vessel wall can occur both in pathological conditions of the whole organism (acidosis, hypoxia), and in direct damage to the vessel wall by biologically active agents. The role of such agents is inflammatory mediators in vasculitis (inflammation of the vascular wall).
If the damage progresses, there is leakage (diapedesis) of red blood cells from the blood into surrounding tissues and the formation of hemorrhages.
Intravascular (intravascular) disorders
The main intravascular disorders are indicated in Fig. 1
Intravascular microcirculation disorders
Slowing of blood and/or lymph flow; excessive acceleration of blood and/or lymph flow; Violation of turbulence of blood and/or lymph flow. Excessive increase in juxtacapillary blood flow
Rice. 1 – Intravascular microcirculation disorders
Reasons for slowing blood and/or lymph flow:
- disorders of hemo‑and lymphodynamics (for example, with heart failure, venous hyperemia, ischemia, lymphorrhea);
- increased blood viscosity (for example, as a result of hemoconcentration during prolonged vomiting, diarrhea, plasmorrhagia due to burns, polycythemia, hyperproteinemia, intravascular disseminated blood coagulation);
- reduction in the lumen of microvessels (due to compression of them by a tumor, edematous tissue, formation of blood clots in them, embolism, swelling or hyperplasia of endothelial cells, formation of atherosclerotic plaque, etc.).
Reasons for excessive acceleration of blood and/or lymph flow:
- disorders of hemo- and/or lymphodynamics (for example, with pathological arterial hyperemia or discharge of arterial blood into the venous bed through arteriovenular shunts);
- decreased blood viscosity (with hemodilution, hypoproteinemia, renal failure, pancytopenia.
https://www.youtube.com/watch?v=ytadvertiseru
Causes of blood flow turbulence:
- damage to the walls of microvessels and/or disruption of their smoothness (with vasculitis, endothelial cell hyperplasia, arteriosclerosis, fibrotic changes in the vascular wall, etc.).
- changes in the aggregate state of the blood (for example, during the formation of parietal microthrombi that disrupt laminar blood flow).
Excessive increase in juxtacapillary blood flow
It develops with the opening of arteriovenous and arteriolovenular shunts and is manifested by the discharge of excess blood from arteries and arterioles into veins and venules, bypassing the capillary network of the microcirculatory bed. Causes:
- spasm of arterioles and closure of precapillary sphincters with a significant increase in the level of catecholamines in the blood (with hypercatecholamine crisis in patients with a tumor of the adrenal medulla - pheochromocytoma);
- with an excessive increase in the tone of the sympathetic nervous system (under stress).
Transmural microcirculation disorders are divided into 2 subgroups:
- changes in fluid flow;
- movement of blood cells.
In pathology, an increase or decrease in the intensity of the transition of substances through the vascular wall is often observed, not only as a result of changes in the intensity of blood flow, but also as a result of a true violation of vascular permeability, which is accompanied by a change in the structure of the wall of metabolic vessels.
Exchange vessels are characterized by two main phenomena - the movement of blood and the ability to pass water, soluble gases and large-molecular (protein) substances in the BLOOD-TISSUE direction and back. The morphological basis of permeability is the endothelium and basement membrane. These formations, as well as the perivascular connective tissue, form a histohematic barrier.
The mechanism of passage of a substance through the vascular wall can be active or passive.
Active action is carried out against concentration and electrochemical gradients, and its implementation requires a fairly large amount of energy (proteins and other macromolecules).
Passive transport (transfer of water, soluble gases and low molecular weight substances) transport occurs in accordance with concentration and electrochemical gradients.
Extravascular disorders
Pathological processes in the body can affect microcirculation vessels in two ways:
- The reaction of tissue basophils, which release into the environment biologically active agents and enzymes that directly affect the vessel and thicken the blood in the vessels.
- Impaired transport of tissue fluid.
Thus, microcirculation is a complex system that is in constant interaction with the entire body. It is necessary to know not only the main types of its disorders, but also methods for diagnosing and treating these diseases.
Are microcirculatory disorders dangerous?
Undoubtedly, many microcirculation disorders are dangerous to the health and even life of the patient, primarily if they occur acutely. Thus, disturbances in blood flow in small vessels of the heart muscle that occur during acute coronary thrombosis lead to severe myocardial ischemia, and after a few minutes or hours - to necrosis (death) of heart muscle cells - acute myocardial infarction develops. The wider the affected area, the more unfavorable the prognosis.
In acute thrombosis of the femoral arteries and veins, any delay in terms of medical and surgical intervention can lead to the loss of a limb.
The same applies to persons with diabetic angiopathy and diabetic foot syndrome. Such patients should be taught how to properly care for their feet so as not to lose their legs if a purulent infection or gangrene of the foot develops.
In the case of long-term processes in the body, for example, with microcirculation disorders in the kidneys and brain with hypertension, there is, of course, a dysfunction of the organ, but there is no acute threat to life.
Age-related disorders of blood flow in the microvessels of the skin do not pose any danger to life and health at all, but only cause aesthetic problems.
Microhemodynamic disorders: diagnosis
Depending on the affected organ, various instrumental diagnostic methods can be used, which can indirectly indicate the presence of microcirculation disorders through the pathology of the internal organ:
- electrocardiogram, echocardiogram, coronary angiography (myocardium);
- Ultrasound of head and neck vessels, Dopplerography, angiography (brain);
- Ultrasound, glomerular filtration rate, excretory urography (kidneys);
- Ultrasound, angiography, capillaroscopy, phlebography (lower limbs).
Microhemodynamic disorders: treatment
To improve microcirculation, a group of drugs called angioprotectors is used. These are highly effective medications that improve blood flow through vessels and restore the vessel itself. Their main properties are:
- reducing arterial spasm;
- ensuring vessel patency;
- improvement of blood rheology (viscosity);
- strengthening the vascular wall;
- anti-edematous effect;
- improving metabolism, that is, metabolism, in the vascular wall.
The main drugs that improve microcirculation include the following:
- "Troxevasin";
- "Detralex";
- "Trental";
- "Emoxipin";
- "L-lysine aescinate."
We can conclude that, despite their small size and diameter, microhemodynamic vessels perform a very important function in the body. Therefore, microcirculation is a self-sufficient system of the body, the condition of which can and should be given special attention.
Prevention of circulatory disorders
In most diagnosed cases, problems with blood vessels appear due to an incorrect lifestyle.
To maintain tone and improve the condition of the cardiovascular system, doctors recommend: Be sure to exercise, spending up to 150 minutes on active cardio exercises every week. Exercise bikes, brisk walking, dancing and swimming are beneficial. To tone blood vessels, use contrasting douches on the limbs or the whole body, sometimes take hot baths with a temperature of 40° C. Give up bad habits. Nicotine causes severe spasms of capillaries, provokes their fragility, narrowing, and increases circulatory disorders. Eat right, don’t forget about healthy saturated fats, Omega-3 and Omega-6 amino acids. calcium, potassium and selenium. When blood circulation changes, disturbances affect the entire body. At the initial stage, problems are eliminated with the help of physical therapy, a special diet, and control of cholesterol and blood sugar levels. If you apply late, you will have to deal with chronic diseases and serious pathologies.