The combination of four strictly defined anatomical defects in the structure of the heart and blood vessels gives grounds to diagnose a congenital heart defect called tetralogy of Fallot.
The disease was identified and described by the French pathologist E.L. Fallot in 1888. Over the past 100-plus years, doctors have achieved good results in the early detection and elimination of this defect.
What is it: description and code according to ICD-10
Tetralogy of Fallot is a congenital heart defect that is quite often diagnosed in newborns. The ICD-10 code is Q21.3.
Tetralogy of Fallot occurs in 3% of children , which is a fifth of all detected congenital heart defects. 30% of all miscarriages and non-developing pregnancies are associated with the presence of tetralogy of Fallot in the fetus, and another 7% of babies with this pathology are stillborn.
These sad figures do not at all indicate the absolute lethality of this disease, but draw attention to its severity and the inevitability of urgent medical intervention.
Tetralogy of Fallot is diagnosed if there is a combination of four anatomical defects in the structure of the cardiovascular system in a newborn:
- Stenosis or narrowing of the pulmonary artery , which leaves the right ventricle. Its immediate purpose is to conduct venous blood to the lungs. If there is a narrowing at the mouth of the pulmonary artery, then blood from the ventricle of the heart enters the artery with force. This leads to a significant increase in the load on the right side of the heart. With age, stenosis intensifies - that is, this defect has a progressive course.
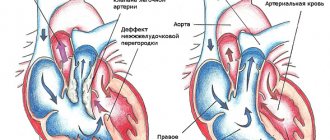
- Defect in the form of a hole in the interventricular septum (VSD) . Normally, the ventricles of the heart are separated by a solid septum, which allows them to maintain different pressures. With tetralogy of Fallot, there is a gap in the interventricular septum and therefore the pressure in both ventricles is equalized. The right ventricle pumps blood not only into the pulmonary artery, but also into the aorta.
- Dextraposition or displacement of the aorta . Normally, the aorta is located on the left side of the heart. In tetralogy of Fallot, it is displaced to the right and is located directly above the hole in the interventricular septum.
- Enlargement of the right ventricle of the heart . It develops secondarily due to increased load on the right side of the heart due to narrowing of the artery and displacement of the aorta.
In 20-40% of cases with tetralogy of Fallot, there are concomitant heart defects:
- right-sided aortic arch;
- hole in the interatrial septum;
- patent ductus arteriosus;
- accessory superior vena cava on the left.
Comparison table of triad, tetrad and pentad of Fallot.
| Disease | Triad | Tetrad | Pentad |
| Anatomical defects |
|
| Similar to tetralogy, but additionally there is a fifth defect - atrial septal defect. |
Prenatal diagnosis of tetralogy of Fallot
Ultrasound scanner WS80
An ideal tool for prenatal research.
Unique image quality and a full range of diagnostic programs for an expert assessment of a woman’s health.
Tetralogy of Fallot is characterized by underdevelopment of the outflow tract of the right ventricle and displacement of the conical septum anteriorly and to the left. Displacement of the septum causes stenosis of the outflow tract of the right ventricle with impaired development of the fibrous ring of the pulmonary trunk, its valve apparatus and, very often, the pulmonary artery trunk.
The first reports of the defect date back to 1671. In 1888, Fallot systematized the observations and described the defect as an independent nosological form, giving an anatomical description of the defect, which was later named after him. It accounts for 12-14% of all congenital heart defects (G. Buncle, 1980) [1]. These figures are based on pathological and anatomical material. In 1999, C. Tennstedt et al. [2] based on 815 sections of congenital malformations in the fetus and newborn showed that heart defects in 31% of cases occur in spontaneous and infected abortions and in 7% of cases are observed in stillborns. In the clinic, tetralogy of Fallot accounts for only 3%.
The severity and prognosis are determined by the degree of stenosis of the right ventricular outflow tract. Moreover, 25% die within a year of life, mainly in the first months, 40% - by 3 years of life, 70% - by 10 years of life and 95% - by 40 years of life (V.I. Burakovsky, L.A. Bockeria, 1989 [3]). In 10-20% there is various extracardiac pathology (H. Bankle, 1970) [4], but no characteristic combinations were noted.
Isolated reports describing the diagnosis of tetralogy of Fallot are mainly represented by works on screening ultrasound detection of heart defects in the fetus without analysis of characteristic echocardiographic features or with descriptions of isolated cases (CS Kleimman et al., 1982 [5]; LD Allan et al., 1984, [ 6]; JW Vladimiroff et al., 1984 [7]; JA Copel et al., 1987 [8]; RG Williams, 1989 [9]; DL Brown et al., 1993 [10]; CC Liang et al., 1997 [11]; SJ Yoo et al., 1997 [12]).
Analysis of prenatal and postnatal ultrasound examinations of 18 observations of patients with tetralogy of Fallot formed the basis for writing this work. In 3 cases the pregnancy was terminated; 15 newborns were born with normal weight and height parameters. On the first day, the condition of the newborns was satisfactory without any clinical manifestations of heart pathology. After functional closure of the ductus arteriosus, a significant deterioration in the condition of the children was noted, and therefore they underwent a detailed examination, including a comprehensive cardiological examination. Due to the discovery of a congenital heart defect, the newborns were transferred to a specialized cardiology department for treatment and preparation for surgery. The fate of the children was tracked throughout the year. There were 6 deaths, 5 observations of effective cardiac surgical treatment, and in 4 cases the condition of the children remained severe with signs of circulatory decompensation, which is why they did not undergo surgical intervention.
Analysis of the results of ultrasound examination during prenatal examination revealed certain patterns. In all cases, ultrasound examination revealed a defect of the interventricular septum (Fig. 1), which was most clearly visualized not when obtaining a four-chamber section of the heart, but when scanning along the long axis of the heart from the cavity of the left ventricle to the aorta, proving a violation of the septal-aortic continuation.
Rice. 1.
Scanning the heart along the long axis in a newborn - 2 days of life. There is a significant expansion of the aorta (AO), its displacement anteriorly, due to which the aorta is located as a “rider aorta”. There is marked hypertrophy of the interventricular septum (IVS) and myocardium of the posterior wall of the left ventricle (LV). RV - right ventricle. The arrow indicates a high ventricular septal defect.
The aortic diameter in all patients, both antenatally and in the early neonatal period, exceeded normal values. The aorta in all cases was displaced in relation to the interventricular septum. The severity of aortic dextroposition varied from slight to severe (see Fig. 1).
A narrowing of the pulmonary artery at the level of the valve ring from minor to complete obstruction was revealed. The most informative method for assessing the degree of narrowing was the use of color flow mapping (Fig. 2, 3).
Rice. 2.
Newborn - 2 days of life. Scanning of the outflow tract of the right ventricle and the trunk of the pulmonary artery (AP). There is a narrowing at the level of the valve ring to 0.48 cm (normal 0.9-1).
Rice. 3.
Color mapping of right ventricular outflow tract flow in a fetus at 31 weeks of gestation. There is a narrowing at the level of the valve ring (arrow).
All of the above ultrasound signs are the main ones in the diagnosis of tetralogy of Fallot for any age and are classic, the description of which has been devoted to many publications since 1972 (H. Feigenbaum, 1972 [13]; F. Vernejoul et al., 1977 [14]; V.V. Bobkov et al., 1978 [15], V.I. Burakovsky, L.A. Bockeria, 1989 [3]).
Analysis of the material showed that, along with these classic manifestations of the defect in the prenatal period, there are ultrasound manifestations of pathology that differ from the classics. The increased size of the left ventricle, in contrast to the norm, was noteworthy. As is known, antenatally and postnatally the size of the left ventricle is either equal to the size of the right ventricle or slightly smaller than it. In all observations, the left ventricle was larger in cavity size than the right ventricle (Fig. 4).
Rice. 4.
Long axis scanning in a newborn. The aorta (AO) is dilated and displaced. The left ventricular (LV) cavity is enlarged. The right ventricle (RV) is small in size. LA - left atrium. Arrow—ventricular septal defect.
In absolute terms, the size of the right ventricle was less than normal for each stage of pregnancy or for a certain day of life, but the degree of reduction in the size of the right ventricle varied within different limits. In addition, there was pronounced hypertrophy of the myocardium of the posterior wall of the left ventricle and the interventricular septum, starting from 22 weeks. gestation, which also differs significantly from normal fetal heart parameters (Fig. 5).
Rice. 5.
Ultrasound examination of the fetal heart at 28 weeks of pregnancy. There is marked myocardial hypertrophy and an increase in the size of the left ventricle (LV) and interventricular septum (IVS). There is narrowing of the pulmonary artery (PA) at the level of the valve annulus and aneurysmal dilation of the truncus (arrow), indicating severe valvular stenosis.
Also, not a single observation revealed infundibular narrowing in the right ventricle. Only a small proportion of patients showed increased trabecularity of the right ventricle.
Thus, according to the results of ultrasound examination in the prenatal period, the following characteristic signs were noted:
- reduction in the absolute size of the right ventricle;
- absence of right ventricular myocardial hypertrophy;
- increased size of the left ventricle;
- hypertrophy of the left ventricular myocardium and interventricular septum;
- the presence of a high ventricular septal defect;
- expansion of the aortic root;
- anterior displacement of the aorta or “ridden aorta.”
In the postnatal period, the use of Doppler mapping adds the following ultrasound signs to the diagnosis of pathology, due to the nature of the disturbance of intracardiac hemodynamics in tetralogy of Fallot:
- turbulent flow in the right ventricle during systole due to a shunt from the left to the right ventricle;
- turbulent flow in the outflow tract of the left ventricle in diastole due to shunting of blood from the right to the left ventricle;
- increased blood flow velocity and turbulent flow in the pulmonary artery due to the presence of narrowing.
The obtained ultrasound signs were compared with clinical manifestations, surgical or autopsy data. It turned out that the degree of reduction in the size of the right ventricle, as well as hypertrophy of the left ventricular myocardium, an increase in its size and hypertrophy of the interventricular septum, are directly dependent on the severity of obstruction at the level of the pulmonary artery valve ring.
Discussion
Over the years, fetal cardiology has devoted a small number of publications to the prenatal diagnosis of tetralogy of Fallot (CS Kleinmann et al., 1982 [5]; LD Allan et al., 1984 [6]). Statistical data are most often cited in general works on the diagnosis of congenital heart defects. (SJ Yoo et al., 1997[12]; JM Schleich et al.,1998[16]).
The introduction of new modifications of ultrasound examination gave a new impetus to the appearance in 1998-99. several articles devoted to the diagnosis of this pathology. (T. Ohba et al., 1990 [17]; CC Hsieh et al., 1995 [18]; LK Hornberger et al., 1995 [19]; SJ Yoo et al, 1999 [20]).
The authors are unanimous in the opinion that with routine ultrasound examination, the diagnosis of tetralogy of Fallot is difficult, since four chambers of the heart and three great vessels are clearly visualized in cross section (SJ Yoo et al, 1997 [12]). Therefore, in publications devoted to the antenatal diagnosis of tetralogy of Fallot, many authors focus on the fact that the leading signs of pathology, which are constantly encountered, are considered to be the presence of a large ventricular septal defect and the obligatory presence of three great vessels when examined along the short axis (CC Hsieh et al., 1995 [18]; SJ Yoo et al., 1997 [12]). Thus, the presence of a high ventricular septal defect, dilatation of the aorta and its displacement, as well as the mandatory presence of three great vessels are differential diagnostic criteria for tetralogy of Fallot in contrast to the common truncus arteriosus and transposition of the great vessels. As diagnostic criteria, the authors proposed (LK Hornberger et al., 1995 [19]) to evaluate in dynamics the ratio of the diameter of the pulmonary artery to the diameter of the aorta, which in the group of patients with tetralogy of Fallot is 0.50-0.73±0.15, depending on degree of severity of pathology. At the same time, the authors believe that only dynamic observation allows one to evaluate this ratio, since at 23-24 weeks of gestation it can be normal. As for infundibular (subvalvular) stenosis, which is included in the diagnostic signs of the pathology, it is not detected in the early stages either by ultrasound or section (T. Ohba et al., 1990 [17]). This corresponds to the opinion prevailing in the literature that this component of the pathology manifests itself at a more mature age and increases with the patient’s age (V.I. Burakovsky, L.A. Bockeria, 1989 [3]).
The presented work shows that the main differential diagnostic feature that allows an antenatal diagnosis of tetralogy of Fallot is hypertrophy of the interventricular septum, left ventricular myocardium and its increased size. Hypertrophy of the left ventricular myocardium in this pathology is due to the characteristics of blood circulation, since tetralogy of Fallot is one of the few congenital heart defects that cause disturbances in intracardiac hemodynamics antenatally. In the presence of severe stenosis or atresia of the pulmonary artery, pulmonary blood flow, in contrast to the norm, is carried out from the left ventricle through the ductus botallus (K. Jefferson et al., 1972 [21]). Due to preserved blood circulation, fetal development does not suffer, and children are born at term with normal weight and height. Since the right ventricle in the presence of this form of the defect is not involved in work, then, unlike the norm, its hypertrophy is not observed antenatally, as shown in the presented work, as well as by other authors (R. Romero et al., 1994 [22]) . At the same time, hypertrophy of the left ventricular myocardium is observed as it takes over the function of the right ventricle. Antenatal diagnosis of one of the main signs of pathology - ventricular septal defect, according to our data, should be carried out not with a four-chamber section, but along the long axis of the heart.
After birth, hemodynamic changes in tetralogy of Fallot are determined by the degree of obstruction to the ejection of blood from the right ventricle into the pulmonary circulation and the presence of a ventricular septal defect.
Thus, analysis of antenatal and postnatal changes in echocardiographic parameters revealed their features in tetralogy of Fallot, thereby facilitating the correct diagnosis of one of the most common congenital heart defects.
Literature
- Buncle G. Congenital defects of the heart and large vessels. - M.: Medicine, 1980.
- Tennstedt C., Chaoui R., Korner H., Dietel M. Spectrum of congenital heart defects and extracardiac malformations associated with chromosomal abnormalities: results of a seven year necropsy study // Heart, 1999, v.82, N1, p.34 -39.
- Burakovsky V.I., Bockeria L.A. Cardiovascular surgery. - M.: Medicine, 1989.
- Bankle H. Das konnatale Herzvitium in der Sektionsstatistik //Arch. Kreisl. Forst., 1970., v.62.118.
- Kleimman CS, Donnerstein RL, De Vore GR et al. Fetal echocardiography for evaluation of in utero congestive heart failure: A technique for study of nonimmune fetal hydrops // N.Engl.J.Med. 1982.-306.- p.568.
- Allan LD, Crawford DC, Anderson RH et al. Echocardiography and anatomical correlations in fetal congenital heart disease // Br. Heart J. 1984.52.-p.542.
- Vladimiroff JW, Stewart PA Tonge HM The role of diagnostic ultrasound in the stydy of fetal cardiac abnormalities // Ultrasound Med. Biol.1984.-10.-p.457.
- Copel JA, Pilu, Green J et al. Fetal echocardiographic screening for congenital disease: The importance of the four chamber views // Am. J.Obstet..-1987.-157.p.648.
- Williams RG Fetal cardiology //Current Opinion in Cardiology.-1989, .-N4.-p.60-63.
- Brown DL, Disalvo DN, Frates MC et al. Sonography of the fetal heart: normal variants and pitfalls // AJR. Am. J. Roentgenol., 1993, v.160, N6.p.1251-1255.
- Liang CC, Tsai CC, Hsieh C., Soong YK Prenatal diagnosis of tetralogy of Fallot with absent pulmonary valve accompanied by hydrops fetalis // Gynecolol. Obstet. Invest, 1997, v.44.N1, p. 61-63.
- Yoo SJ, Lee YH, Kim ES, Ryu HM, Kim MY, Choi HK, Cho KS, Kim A. Three-vessel view of the fetal upper mediastinum: an easy means of detecting abnormalities of the ventricular outflow tracts and great arteries during obstetrics screening // J. Ultrasound Obstet Gynecol, 1997, v.9, N3, p. 173-82.
- Feigenbaum H. Echocardiography.// Philadelphia, lea, Febiger, 1972.
- Vernejoul F., Baligadao S., Haiat R. Progress recents en echocardiographie. //Coeur Med. Interne., 1977, v.16, N1, p. 139-152.
- Zaretsky V.V., Bobkov V.V., Olbinskaya L.I. Clinical echocardiography. - M.: Medicine, 1979.
- Schleich JM, Almange C. Fetal echocardiography // Arch Pediatr., 1998, v.5, N11, p. 1236-1245.
- Ohba T., Matsui K., Nakamura K., Nishimura K., Ito M., Okamura H. Tetralogy of Fallot with absent pulmonary valve detected by fetal echocardiography // Int. Gynaecol. Obstet., 1990, v.32., N1, p.71-74.
- Hsieh CC, Chiu TH, Kuo DM, Hsieh TT Prenatal diagnosis of tetralogy of Fallot by Doppler color flow mapping // J. Formos Med. Assos., 1995, v.94., N10, p. 619-621.
- Hornberger LK, Sanders SP, Sahn DJ, Rice MJ, Spevak PJ et al. In utero pulmonary artery and aortic growth and potential for progression of pulmonary outflow tract obstruction in tetralogy of Fallot. // J. Am. Coll.Cardiol., 1995, v.25, N3.p.739-745.
- Yoo SJ, Lee YH, Kim ES, Ryu HM, Kim MY, Chun YK, Hong SR Tetralogy of Fallot in the fetus findings at targeted sonography // Ultrasound Obstet. Gynecology, 1999 v. 14, N1, p.29-37.
- Jefferson K., Rees S., Somerville J. Systemic arterial supply to the lung in pulmonary atresia and its relation to pulmonary artery development // Br.Heart J.-1972.-v.34.-p.418.
- Romero R., Pilu V., Genti F., Ghidini A., Hobbins D. Prenatal diagnosis of congenital malformations of the fetus. - M.: Medicine, 1994.
Ultrasound scanner WS80
An ideal tool for prenatal research.
Unique image quality and a full range of diagnostic programs for an expert assessment of a woman’s health.
Hemodynamics
With the defect, the following sequential hemodynamic disturbances develop:
- Due to narrowing of the pulmonary artery, the right ventricle experiences volume overload (from the right atrium) and pressure (from the pulmonary artery). The overflow of blood causes an increase in its striking force;
- When the pressure in the right ventricle becomes critical, venous discharge begins from right to left;
- From the left ventricle mixed blood enters the aorta;
- The aorta, due to congenital dextroposition, additionally receives blood from the right ventricle;
- The content of carbon dioxide in the blood increases, oxygen decreases (hypoxemia);
- Hypoxia begins in the internal organs, which is aggravated by depletion of the pulmonary circulation;
- Due to oxygen deficiency, polycythemia develops - an increase in the level of blood cells and hemoglobin.
Useful video that talks about hemodynamics in tetralogy of Fallot:
Operation technique
To perform an anastomosis, the chest is opened using a right-sided anterior approach in the third intercostal space.
The pericardium is opened longitudinally and the right branch of the pulmonary artery is isolated in the space between the aorta and the superior vena cava. Using a Satinsky clamp, the aorta and the proximal end of the right branch of the pulmonary artery are simultaneously squeezed parietally. Then the distal end of the artery is clamped with a tourniquet. Holes with a diameter of more than 3-4 mm are made in the wall of the aorta and artery and the anastomosis is performed with a continuous suture. The advantages of the operation are undeniable. In terms of efficiency, it is equal to the Potts anastomosis, however, it does not require dissection of a large array of muscles, the mediastinal collaterals are not separated, and it is equally easy to perform with a right- and left-oriented aortic arch and in any age group of patients. When performing a radical operation, the superimposed anastomosis can be easily sutured during artificial circulation with access through the aorta.
Among the group of palliative operations aimed at eliminating stenosis, only pulmonary valvotomy and resection of infundibular stenosis with access through the right ventricle have stood the test of time (Brock, Sellors, 1948).
Causes and risk factors
The anatomical structures of the heart are formed in the fetus in the first three months of intrauterine development .
It is at this time that any damaging external influences on a pregnant woman’s body can have a fatal impact on the formation of congenital heart disease.
Risk factors at this time are:
- viral diseases occurring in acute form;
- use of certain medications, psychoactive and psychotropic substances (including tobacco and alcohol) by a pregnant woman;
- heavy metal poisoning;
- radioactive exposure;
- The age of the expectant mother is more than 40 years.
European scientists have discovered that tetralogy of Fallot correlates with some genetic diseases in infants, which allowed them to assume the hereditary nature of this defect.
Reasons for development
The most important etiological factors in the development of this pathological condition are:
- Rubella, scarlet fever, measles, which a pregnant woman suffered in the early stages of pregnancy.
- The use of drugs from the group of hypnotics, hormonals, and sedatives.
- Alcohol consumption.
- Taking drugs.
- Smoking.
- Exposure to harmful substances.
- Heredity.
- Cornelia de Lange syndrome (dwarfism, which includes mental retardation, ear deformation, gothic palate, choanal atresia, “clown face”, myopia, strabismus, astigmatism, hypertrichosis, optic atrophy).
- Late pregnancy (after 40 years) with concomitant diabetes mellitus, systemic lupus erythematosus, and other systemic diseases.
Important! The formation of such a congenital pathology as tetralogy of Fallot primarily occurs at 2–8 weeks of embryogenesis.
Types of congenital heart disease and stages of its development
Depending on the anatomical features of the defect, several types of tetralogy of Fallot are distinguished:
- Embryological - the maximum narrowing of the artery is at the level of the demarcating muscle ring. The stenosis can be corrected by sparing resection of the conus arteriosus.
- Hypertrophic - the maximum narrowing of the artery is at the level of the demarcating muscle ring and the entrance to the right ventricle. The stenosis can be corrected with massive resection of the conus arteriosus.
- Tubular - the entire arterial cone is narrowed and shortened. Patients with this defect cannot undergo radical surgical correction of pulmonary artery stenosis. For them, palliative plastic surgery is preferable to prevent the worsening of the disease.
- Multicomponent - arterial stenosis is caused by a number of anatomical changes, the position and characteristics of which determine the successful outcome of surgical correction of stenosis.
Depending on the characteristics of the clinical manifestations of the defect, the following forms are distinguished:
- cyanotic or classic - with pronounced cyanosis (blueness) of the skin and mucous membranes;
- acyanotic or “pale” form - more common in children in the first 3 years of life as a result of partial compensation of the defect.
Depending on the severity of the disease, the following forms of the disease are distinguished:
- Heavy . Shortness of breath and cyanosis appear almost from birth during crying and feeding.
- Classic . The disease debuts at the age of 6-12 months. Clinical manifestations closely correlate with increased physical activity.
- Paroxysmal . The child suffers from severe dyspnea-cyanotic attacks.
- Mild form with late onset of cyanosis and shortness of breath - at 6-10 years.
In its course, the disease goes through three successive stages:
- Relative well-being . Most often, this phase lasts from birth to 5-6 months. There are no pronounced symptoms of the disease, which is due to partial compensation of the defect due to the structural features of the newborn’s heart.
- Cyanotic phase . The most difficult period in the life of a child with tetralogy of Fallot lasts 2-3 years. At the same time, all clinical manifestations of the disease are clearly expressed: shortness of breath, cyanosis, attacks of suffocation. This age group accounts for the greatest number of deaths.
- Transitional phase or phase of formation of compensatory mechanisms. The clinical picture of the disease remains, but the child adapts to his illness and is able to prevent or weaken attacks of the disease.
Symptoms of heart disease
Cyanotic skin color is the most typical sign of Fallot's defects. Depending on the time of its appearance, 5 clinical variants of the disease are identified:
- early cyanotic (from 1 to 12 months);
- classic (child 2 - 3 years old);
- severe (attacks of cyanosis, shortness of breath);
- late cyanotic (6 - 10 years);
- acyanotic (pale skin).
A severe variant of tetralogy of Fallot occurs at 3–4 months and increases in severity by the age of one year. The child turns blue when eating, crying, or exercising. Any type of activity causes weakness, difficulty breathing, dizziness, and palpitations. After running or active games, the child squats down to recover.
Attacks of cyanosis become extremely severe from the second to the fifth year of life, they are accompanied by the sudden onset of weakness, anxiety, and loss of consciousness. Convulsions and coma may occur in the absence of breathing.
These signs are caused by spasm of the right ventricle, the complete flow of venous blood into the arterial network, which causes oxygen starvation of the brain.
Children with Fallot's defects begin to walk later, are delayed in development, and suffer from colds and pneumonia more often than their peers. Adult patients are at high risk of developing tuberculosis.
Danger and complications
The diagnosis of tetralogy of Fallot is classified as a severe heart defect with high mortality. If the course of the disease is unfavorable, the average life expectancy of the patient is 15 years .
The following complications of the disease are common:
- thrombosis of blood vessels in the brain or lungs due to increased blood viscosity;
- brain abscess;
- acute or congestive heart failure;
- bacterial endocarditis;
- delayed psychomotor development.
Complications
After cardiac surgery, complications may develop in the form of:
- Preservation of pulmonary stenosis.
- Development of heart rhythm disturbances.
Complications that develop due to hypoxia:
- Infectious endocarditis.
- Brain abscesses.
- Hypovitaminosis.
- Iron-deficiency anemia.
Important! Without surgery, a child’s life expectancy rarely reaches 12–13 years.
Symptoms of the disease
Depending on the degree of narrowing of the artery and the size of the hole in the interventricular septum, the age at which the first symptoms of the disease appear and the degree of their progression changes. Let's look at the clinical picture of the disease; tetralogy of Fallot is characterized by:
- Cyanosis . Most often it appears in children under the age of one year, first on the lips, then on the mucous membranes, face, arms, legs and torso. Progresses as the child’s physical activity increases.
- Thickened fingers in the form of “drumsticks” and convex nails in the form of “hour glasses” are formed by the age of 1-2 years.
- Shortness of breath in the form of deep arrhythmic breathing. At the same time, the frequency of inhalations and exhalations does not increase. Shortness of breath worsens with the slightest physical exertion.
- Fast fatiguability.
- The cardiac hump is a bulge on the chest in the area of the heart.
- Delayed motor development due to forced restrictions in physical activity.
- Heart murmurs.
- The typical body position of a sick child is squatting or lying down with legs bent to the stomach. It is in this position that the baby feels better and therefore unconsciously accepts it as often as possible.
- Fainting up to the state of hypoxemic coma.
- “Blue” attacks of shortness of breath and cyanosis during a severe paroxysmal course of the disease, in which young children (1-2 years old) suddenly turn blue, begin to choke, become restless, and then may lose consciousness or even fall into a coma. After the attack, the child was lethargic and drowsy. Often, as a result of such an exacerbation, the baby may even die.
If you suspect tetralogy of Fallot, you should consult a doctor immediately. The child requires emergency consultation with a cardiologist or cardiac surgeon.
Dyspnea-cyanotic attack
The attack develops with a significant narrowing of the pulmonary trunk and is typical for young children . Caused by spontaneous contraction of the right ventricle, which worsens hypoxemia. Oxygen deficiency provokes brain hypoxia, and excess carbon dioxide stimulates the respiratory center.
The attack is manifested by sudden inspiratory shortness of breath (difficulty and increased inhalation). The child's breathing resembles that of a foreign body and is accompanied by a generalized bluish discoloration of the skin. The patient takes a forced squatting position and loses consciousness within 1-2 minutes.
Algorithm of actions:
- Call an ambulance.
- Protect the child from strangers.
- Provide fresh air flow.
- Unbutton your clothes.
- Make sure the airway is clear.
Upon arrival of the ambulance, emergency measures are taken:
- oxygen therapy through a mask (20% moist oxygen);
- determination of vital signs;
- administration of bronchodilators and cardiotonics (aminophylline, norepinephrine);
- taking an ECG and simultaneous preparation for the administration of antiarrhythmics (the attack is often complicated by fibrillation);
- transportation to a hospital facility.
All drugs are administered intravenously. While providing assistance, paramedics keep a defibrillator at the ready. In the hospital, the child’s condition is stabilized with heart medications and oxygen therapy.
Symptoms
The clinical picture appears:
- significant cyanosis - located around the lips, in the upper half of the body, intensifies when the child cries, feeds, strains;
- shortness of breath - has a paroxysmal nature associated with physical activity, the child takes the most comfortable “squatting” position, due to a temporary reflex additional spasm of the pulmonary artery and the cessation of blood oxygen saturation by 2 times;
- fingers in the shape of “drum sticks”;
- physical underdevelopment and weakness of children; running, outdoor games cause increased fatigue, dizziness;
- convulsions - associated with hypoxia of brain structures, blood thickening, and a tendency to thrombosis of cerebral vessels.
The form of the disease depends on the age of the child, and it determines the adequacy of compensation; in a newborn, cyanosis is visible on the face, hands and feet
There are:
- early manifestations in the form of cyanosis immediately after birth or in the first 12 months of life;
- The classic course is considered to be the manifestation of cyanosis at two to three years of age;
- severe form - paroxysmal clinical picture with shortness of breath and cyanosis;
- late - cyanosis appears only by 6 or 10 years;
- acyanotic form.
An attack of shortness of breath can occur at rest: the child becomes restless, cyanosis and shortness of breath intensify, the heart rate increases, loss of consciousness with convulsions and subsequent focal manifestations in the form of incomplete paralysis of the limbs is possible.
Diagnosis of the disease
The disease is diagnosed based on anamnesis, after a clinical examination and auscultation of the chest, taking into account the results of instrumental research methods.
Inspection:
- Universal blue discoloration of the skin and mucous membranes throughout the body.
- Growth retardation, dystrophy 2-3 degrees.
- Heart hump.
- Fibrous changes in the fingers and nails (“watch glasses”, “drumsticks”).
Palpation:
- In the projection of the pulmonary artery (the level of the second intercostal space on the left of the sternum), systolic tremor is determined.
- A cardiac impulse is detected in the costal angle.
Auscultation:
- A continuous systolic murmur is heard in the 2nd intercostal space on the left.
- The second tone is muted.
- At Botkin's point there is a soft systolic murmur caused by the discharge of blood through a septal defect.
Laboratory data:
- Polycythemia (increased levels of blood cells and hemoglobin).
- Slowing down of ESR to 2-0 mm per hour.
X-ray:
- Decreased blood supply to the lungs (pallor of the roots).
- Flattening of the pulmonary trunk arch.
- Horizontal position of the right ventricle.
- Shift of the aortic arch to the right.
- Emphasized heart waist on the left.
ECG:
- Shift of the heart axis to the right.
- Significant elevation of the R wave in the right leads (V1-V2).
- Possible signs are bifurcation of the R wave, arrhythmias.
Ultrasound:
- Confirmation of all components of the vice.
- Heart in the shape of a “shoe”.
- Discharge of blood from right to left.
- Pressure difference between the ventricles.
From a diagnostic point of view, the most valuable is cardiac ultrasound with Doppler. Invasive research methods such as angiography and cardiac catheterization in newborns are rarely performed, only in case of unsatisfactory quality of results using other diagnostic methods.
Differential diagnosis of tetralogy of Fallot is carried out with diseases similar in clinical manifestations:
- complete transposition of the great vessels;
- Ebstein's anomaly;
- fusion of the pulmonary artery;
- single ventricle.
How is it detected in the fetus?
In most cases (50-70%), the defect is diagnosed after birth.
A large number of diagnostic omissions are due to the insufficient quality of the ultrasound machine (ultrasound diagnostics is the main method of diagnosing the fetus). Comprehensive diagnostics during pregnancy for the presence of tetralogy of Fallot in the fetus includes:
- Genetic counseling with karyotype determination (the defect is often combined with congenital syndromes - Patau, Down).
- Determination of the size of the collar space in the 1st trimester - the size exceeds 3.5 mm.
- An ultrasound scan in the 1st trimester indicates gross disturbances in the formation of the heart (often also in other organs).
- Ultrasound of the fetus in the 2nd trimester - pulmonary stenosis, septal defect, dextroposition of the aorta.
- Color mapping – turbulent blood flow, insufficient venous flow into the pulmonary artery.
Further tactics:
- When diagnosed before 22 weeks, a woman must determine whether she wishes to continue the pregnancy.
- If consent is given, the pregnant woman is observed by a therapist and an obstetrician-gynecologist before birth.
- Delivery is carried out naturally.
- The child comes under the supervision of a neonatologist, after which he is registered with a cardiologist and cardiac surgeon.
- The time of surgery is planned.
The timing of the intervention depends on the degree of narrowing of the pulmonary artery. With significant stenosis, the operation is performed in the first month of life, with moderate stenosis - within 3 years from birth.
Diagnostic methods
To ensure the presence of tetralogy of Fallot, the examination is carried out strictly according to medical protocol. It involves a careful study of the patient’s complaints, the use of physical and laboratory methods. When interviewing the patient, special attention is paid to the presence of shortness of breath during exercise and at rest, blueness of the lips and nail beds, and the level of physical development and fatigue are analyzed. Physical examination involves auscultation to listen for heart sounds and murmurs.
To deeply study the patient’s condition, laboratory and instrumental studies are carried out:
- blood gas analysis or pulse oximetry - to determine the level of oxygen in the blood;
- electrocardiography – it reveals overload and enlargement of the right ventricle;
- chest x-ray – clarifies how enlarged the right ventricle is, records changes in the shape of the heart;
X-ray of tetralogy of Fallot - Ultrasound of the heart - echocardiography helps to visually examine all the structures of the heart;
- MRI - magnetic resonance imaging provides a complete picture of the trunk, main and peripheral branches of the pulmonary artery, as well as the tricuspid valve.
If ultrasound does not allow an accurate diagnosis to be made, cardiac catheterization and angiocardiography are performed. Thin plastic catheters are passed through the large femoral vein into different parts of the heart. Using these tubes, under X-ray control, blood is taken from different parts of the heart, and a contrast agent is injected to visualize the chambers of the heart. Despite the invasiveness of the study, it is absolutely safe.
Treatment methods
Surgical treatment is indicated. The optimal age for surgery is up to 3-5 years . But you still need to grow to this age, having gone through the stage of severe cyanosis and shortness of breath in a child at 1-2 years old.
Quite often, children with tetralogy of Fallot die during severe dyspnea-cyanotic attacks at an early age, if they are not provided with
competent medical care and support:
- prevention and treatment of concomitant diseases (anemia, rickets, infectious diseases);
- prevention of dehydration;
- sedative therapy;
- treatment with adrenergic blockers;
- treatment with antihypoxants and neuroprotectors;
- oxygen therapy;
- maintenance therapy with vitamins and minerals.
The only contraindication to radical surgical correction for tetralogy of Fallot may be severe pulmonary hypertension in newborns.
Otherwise, surgery is the most effective method of treatment. Depending on the anatomical features of the existing defect, preference is given to the method of surgical intervention:
- Palliative surgery is practiced when it is impossible to completely eliminate a heart defect. Necessary to optimize the patient’s quality of life. Most often, an aortopulmonary anastomosis is performed - that is, the pulmonary artery is connected to the subclavian artery using synthetic implants. Sometimes palliative surgery is just the first stage of surgical intervention in a child under 1 year old - it allows the baby to live for several more years before undergoing radical correction of the defect.
- Radical correction involves elimination of the ventricular septal defect and expansion of the pulmonary artery. This is an open heart surgery, after which the child will be able to lead an almost normal life after some time. It is important to note that this operation is recommended for children at least 3-5 years old.
Morphology
Tetralogy of Fallot has four anatomical components:
- ventriculoseptile defect – in this case the ventricular septal defect is non-restrictive and large (perimembranous, muscular, juxta-arterial VSD);
- obstruction of the outgoing portion of the right ventricle - is due to one or more anatomical components, among which are valvular stenosis of the pulmonary artery, obstruction as a result of hypertrophied myocardium of the right ventricle and others;
- dextraposition of the aorta - it partly departs from the right ventricle or blood flow is carried out in it dominantly with the help of the functioning of the left ventricle;
- Right ventricular hypertrophy – usually develops with age.
Stages of surgical treatment
The operation consists of two stages - radical and palliative. Palliative surgery is performed to alleviate the condition of life, radical surgery is performed to eliminate the defect.
The first is elimination of pulmonary stenosis (valvotomy)
It is performed routinely for children under 3 years of age in a cardiac surgery hospital. Access is only open (opening the chest).
Stages:
- Dissection of the ribs and removal of the sternum.
- Opening of the chest cavity and incision of the pericardial sac.
- Insertion of a valvulotome into the heart cavity.
- Dissection of the walls of the pulmonary trunk to expand its diameter.
The result of the operation is to improve the flow of venous blood into the lungs and eliminate hypoxia.
Instead of valvotomy, other interventions may be performed:
- Resection – removal of a narrowed area of the right ventricle in order to increase its cavity;
- Creation of bypass pathways of blood flow - suturing the subclavian artery or aorta to the branches of the pulmonary trunk.
Second – plastic surgery of ventricular septal defect
It is performed when a heart-lung machine is connected 3-6 months after the first intervention.
Stages:
- Opening the chest cavity.
- Connecting a heart-lung machine.
- Cardioplegia (exclusion of the heart from the systemic circulation).
- Compression of the aortic walls.
- Opening of the right ventricle.
- Suturing a septal defect.
- Additional suturing of a patch to the right ventricle to increase the size of its cavity.
Diagnostics
In patients with significant cyanosis in combination with a tendency to bleeding, the following changes are determined:
- Reduced blood clotting factors
- Low platelet count
- Reduced clotting factors
- Decrease in total fibrinogen
- Prolonged prothrombin and coagulation times
When analyzing arterial blood, the following is determined:
- Normal or decreased oxygen saturation
- pH and partial pressure of carbon dioxide (pCO2) are normal unless the patient is critically ill
Of the instrumental research methods used:
- Echocardiography
- Chest X-ray
- Magnetic resonance imaging
- Cardiac catheterization (in extreme cases)
Echocardiography in combination with Doppler provides the following data:
- Accurately diagnoses ductus arteriosus or atrial septal defect
- Coronary lesions are identified with some degree of accuracy
- Valve changes are easily detected
In many institutions, echocardiography is the only diagnostic test used preoperatively.
Video: Tetralogy of Fallot
Chest X-ray provides the following indicators:
- Reduction of vascular pattern in the lungs
- The classic form of “boot heart” (coeur en sabot) is a hallmark of heart disease
MRI allows you to determine:
- Clear location of the aorta, pulmonary artery and its branches
- Condition of the interventricular and interatrial septum, wall thickness of the right ventricle
- The magnitude of intracardiac pressure, gradients and blood flows
Cardiac catheterization may be helpful in any of the following cases:
- The anatomical structure of the heart and accompanying vessels cannot be fully determined by echocardiography
- Existing pulmonary artery disease is a major concern
- Pulmonary or arterial hypertension is determined
Cardiac catheterization may provide results such as the size of the pulmonary annulus and pulmonary arteries, the severity of RV outflow stenosis, and the location and size of associated anomalies and defects.
Prognosis after and without treatment, life expectancy
Without surgery, 3% of patients survive to 40 years of age. Successful surgery allows patients to achieve longevity (70 years or more) . Further observation leads to lifelong registration, monitoring of physical activity, and treatment of concomitant diseases.
Statistics on how long people with this diagnosis live:
- Five-year survival rate after surgery: 92-97%.
- Live up to 20 years: 87-88% of patients.
- Live up to 30 years: 77-80% of patients.
- The average life expectancy after intervention is 66-72 years.
- Life expectancy with concomitant diseases (arrhythmia): 50-55 years.
Clinical guidelines
Recommendations for pregnant women and mothers of children with the defect:
- Early registration for pregnancy (up to 12 weeks).
- Registration of a sick child.
- Exclusion of self-medication and refusal of surgery.
- Minimizing hypothermia, excessive physical activity, and overeating in the child.
Recommendations for patients:
- Monitoring your well-being, weight and sleep duration.
- Ensuring adequate physical activity.
- Balanced diet.
- Classes in the swimming pool, cardiology school.
- Regular medical examination.
- Treatment of concomitant diseases (arrhythmias, blockades).
The only measure to prevent the sad outcome of the disease is a timely visit to the doctor when the first signs of the disease are detected, scrupulous monitoring of the child and vigilant medical supervision. Tetralogy of Fallot is not a disease for which you can experiment with alternative treatments and hope for a miracle. Saving the life of a small patient is only in the hands of a cardiac surgeon.
Antenatal fetal protection or prevention of defects
There is no single method for preventing congenital heart defects in children, including tetralogy of Fallot. There is still a lot of research going on on this issue. Since the condition is present at birth, the expectant mother needs to take some preventive measures during pregnancy to reduce the risk.
- To give up smoking. Smoking during pregnancy is associated with several congenital disorders, including TF. If a woman smokes, she should quit the habit before conceiving to minimize the risk of any problems for the fetus.
- Quitting alcohol. Drinking alcoholic beverages can cause complications in the development of the fetus and is best avoided completely.
- Limited use of medications. Some drugs can cause birth defects in a child. You should always consult your doctor before taking any medications, especially over-the-counter medications.
- Vaccination against rubella and influenza. These infections during pregnancy can cause congenital anomalies in the child, including TF. It is necessary to get vaccinated against rubella six months before conception and against influenza at any time during pregnancy, if there are no contraindications.
- Taking vitamin B9 supplements. Taking 400 mg of vitamin B9 (folic acid) every day during the first trimester reduces the risk of heart defects in the fetus.
- Diabetes control. If a woman has diabetes, her blood sugar levels need to be monitored and controlled. A sharp increase or decrease in blood sugar levels can cause complications in the development of the fetus.
- Avoid exposure to harmful substances: Swallowing or inhaling harmful chemicals may increase the risk of birth defects.