Calcigard retard is a drug (tablets) that belongs to the pharmacological group of calcium channel blockers. Important features from the instructions for use:
How to dissolve vascular plaques, normalize blood circulation, blood pressure and forget the way to the pharmacy
- Sold only with a doctor's prescription
- During pregnancy: contraindicated
- When breastfeeding: contraindicated
- For liver dysfunction: with caution
- If renal function is impaired: with caution
- In old age: with caution
Compound
1 tablet contains:
Tablet core: active substance:
nifedipine 20 mg;
excipients:
lactose monohydrate 20.00 mg, starch 13.44 mg, microcrystalline cellulose 15.00 mg, polysorbate-80 1.00 mg, macrogol 6000 1.00 mg, stearic acid 1.00 mg, povidone (K-30 ) 6.00 mg, magnesium stearate 1.56 mg, sodium lauryl sulfate 2.00 mg; Film coating: hypromellose (15 cps) 2.66 mg, ethylcellulose 0.40 mg, diethyl phthalate 0.26 mg, titanium dioxide 0.26 mg, chocolate brown varnish 0.40 mg, talc 0.02 mg.
Description : Round, biconvex, brown, film-coated tablets with a notch on one side.
pharmachologic effect
Pharmacodynamics
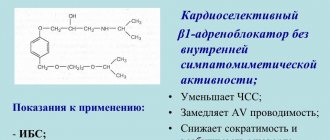
Selective blocker of “slow” calcium channels, a 1,4-dihydropyridine derivative. It has a vasodilating, antianginal and hypotensive effect. Reduces Ca2+ current in cardiomyocytes and smooth muscle cells of coronary and peripheral arteries; in high doses suppresses the release of Ca2+ from intracellular stores. Reduces the number of functioning channels without affecting the time of their activation, inactivation and recovery. It uncouples the processes of excitation and contraction in the myocardium, mediated by tropomyosin and troponin, and in vascular smooth muscles, mediated by calmodulin. In therapeutic doses, it normalizes the transmembrane Ca2+ current, which is disturbed in a number of pathological conditions, primarily in arterial hypertension. Does not affect the tone of the veins. Strengthens coronary blood flow, improves blood supply to ischemic areas of the myocardium without developing the “steal” phenomenon, and activates the functioning of collaterals. By dilating the peripheral arteries, it reduces the total peripheral vascular resistance, myocardial tone, afterload, myocardial oxygen demand and increases the duration of diastolic relaxation of the left ventricle. It has virtually no effect on the sinoatrial and atrioventricular nodes and does not have antiarrhythmic activity. Increases renal blood flow, causes moderate natriuresis. The negative chrono-, dromo- and inotropic effects are overlapped by reflex activation of the sympathoadrenal system and an increase in the number of heart contractions in response to peripheral vasodilation.
The onset of effect is 20 minutes when taken orally; duration of effect – 12-24 hours (prolonged “retard” form).
Pharmacokinetics
Absorption is high (more than 92-98%). Bioavailability – 40-60%. Eating increases bioavailability. Has a “first pass” effect through the liver.
Penetrates the blood-brain barrier and is excreted in breast milk.
Completely metabolized in the liver. The isoenzymes CYP3A4, CYP3A5 AND CYP3A7 are involved in the metabolism of the drug. The half-life (T1/2) is 3.8-16.9 hours. In patients with liver failure, total clearance decreases and T1/2 increases.
There is no cumulative effect. Chronic renal failure, hemodialysis and peritoneal dialysis do not affect pharmacokinetics. With prolonged use, tolerance to the action of the drug develops. Plasmapheresis may enhance elimination.
Analogs
If it is not possible to purchase this drug, then you can always turn to the help of analogues. They are no less effective and have similar properties. Most often, doctors recommend taking instead of Calcigard:
- Adalat is intended for the treatment of hypertension, angina pectoris and cardiac ischemia. Has a hypotensive effect. Belongs to the group of calcium channel blockers. The active component is the same as that of Calcigrad.
- Corinfar - the active substance of the tablets is nifedipine. The medication is recommended for arterial hypertension and angina of any form. Refers to selective calcium channel blockers. It has hypotensive and antianginal properties.
- Cordipine retard - the active ingredient is nifedipine. Prescribed for hypertensive attacks, angina pectoris, Raynaud's syndrome, hypertension of any form. It is often used to prevent angina and hypertensive crises.
- Cordaflex is intended for the treatment and prevention of hypertension of any stage and form, angina pectoris, Raynaud's disease, as well as hypertensive and angina attacks. The active component is similar to Calcigard.
Contraindications, dosages, side effects are almost the same as for Calcigard. Medicines differ only in the form of release and the excipients included in the composition.
Contraindications
Hypersensitivity to nifedipine or other dihydropyridine derivatives, other components of the drug, cardiogenic shock (risk of myocardial infarction), collapse, severe aortic valve stenosis, chronic heart failure (in the decompensation stage), severe arterial hypotension (systolic blood pressure below 90 mm Hg. Art.), acute period of myocardial infarction (during the first 4 weeks), pregnancy (during the first 20 weeks), lactation period, age up to 18 years (efficacy and safety have not been established).
With caution: severe stenosis of the aortic orifice or mitral valve, hypertrophic obstructive cardiomyopathy, severe bradycardia or tachycardia, sick sinus syndrome, malignant arterial hypertension, mild or moderate arterial hypotension, myocardial infarction with left ventricular failure, unstable angina, simultaneous administration of beta-blockers or cardiac glycosides, simultaneous use of rifampcin, severe cerebrovascular accidents, impaired liver and/or kidney function, hemodialysis (risk of arterial hypotension), old age, gastrointestinal obstruction, pregnancy after 20 weeks.
Directions for use and doses
The dosage regimen is set individually depending on the severity of the disease and the response to the therapy. It is recommended to take the drug during or after meals with a small amount of water.
Initial dose: 1 tablet (20 mg) twice a day. If necessary, the dose can be increased. The maximum daily dose of the drug is 120 mg.
In elderly patients or patients receiving combination (antianginal or antihypertensive) therapy, with impaired liver function, in patients with severe cerebrovascular accidents, the dose should be reduced.
Instructions for use
The official instructions for the drug Calcigard retard states that it can be used regardless of food intake. It can be drunk both before and after meals with plenty of water. Dosages are calculated individually depending on the pathology, but as a rule, the daily norm is 1 tablet 2 times a day. If the desired result is not achieved, the dosage can be adjusted, but only in agreement with the cardiologist.
A large amount of water is necessary for better removal of the drug from the body.
Side effect
From the cardiovascular system:
Manifestations of excessive vasodilation (asymptomatic decrease in blood pressure, development or worsening of heart failure, “flushes” of blood to the facial skin, flushing of the facial skin, feeling of heat), tachycardia, palpitations, arrhythmia, peripheral edema, chest pain. Rarely - excessive decrease in blood pressure, fainting; in some patients, especially at the beginning of treatment, angina attacks may occur, which requires discontinuation of the drug; Isolated cases of myocardial infarction have been described.
From the central nervous system:
Headache, dizziness, increased fatigue, weakness, drowsiness. With long-term oral administration in high doses, paresthesia of the limbs, depression, anxiety, extrapyramidal (parkinsonian) disorders (ataxia, “mask-like” face, shuffling gait, stiffness in the movement of arms and legs, tremor of the hands and fingers, difficulty swallowing).
From the digestive system:
Dry mouth, increased appetite, dyspepsia (nausea, diarrhea or constipation). Rarely – gum hyperplasia (bleeding, pain, swelling). With long-term use - liver dysfunction (intrahepatic cholestasis, increased activity of liver enzymes).
From the hematopoietic organs:
Anemia, asymptomatic agranulocytosis, thrombocytopenia, thrombocytopenic purpura, leukopenia.
Allergic reactions:
Rarely – skin itching, urticaria, exanthema, exfoliative dermatitis, photodermatitis. Very rarely – autoimmune hepatitis.
From the musculoskeletal system:
arthritis, arthralgia (rare), joint swelling, myalgia, cramps of the upper and lower extremities.
From the urinary system:
increased daily diuresis, deterioration of renal function (in patients with renal failure).
Other:
Rarely – difficulty breathing, cough; very rarely - visual impairment (including transient blindness at the maximum concentration of nifedipine in the blood plasma), gynecomastia (in elderly patients, completely disappearing after discontinuation of the drug), hyperglycemia, galactorrhea, pulmonary edema, bronchospasm, weight gain.
CALCIGUARD RETARD
Extended-release tablets, brown film-coated
, biconvex, scored on one side.
10 pieces. - blisters (10) - cardboard packs.
Selective class II calcium channel blocker, dihydropyridine derivative. Inhibits the flow of calcium into cardiomyocytes and vascular smooth muscle cells. Has antianginal and hypotensive effects. Reduces the tone of vascular smooth muscles. Dilates coronary and peripheral arteries, reduces peripheral vascular resistance, blood pressure and slightly reduces myocardial contractility, reduces afterload and myocardial oxygen demand. Improves coronary blood flow. It has virtually no antiarrhythmic activity. Does not inhibit myocardial conductivity.
Individual. For oral administration, the initial dose is 10 mg 3-4 times a day. If necessary, the dose is gradually increased to 20 mg 3-4 times a day. In special cases (variant angina, severe arterial hypertension), for a short time the dose can be increased to 30 mg 3-4 times a day. To relieve a hypertensive crisis, as well as an attack of angina, 10-20 mg (rarely 30 mg) can be used sublingually.
IV to relieve an attack of angina or hypertensive crisis - 5 mg for 4-8 hours.
Intracoronary to relieve acute spasms of the coronary arteries, a bolus of 100-200 mcg is administered. For stenosis of large coronary vessels, the initial dose is 50-100 mcg.
Maximum daily doses:
when taken orally - 120 mg, when administered intravenously - 30 mg.
From the cardiovascular system:
hyperemia of the skin, sensation of warmth, tachycardia, arterial hypotension, peripheral edema; rarely - bradycardia, ventricular tachycardia, asystole, increased attacks of angina.
From the digestive system:
nausea, heartburn, diarrhea; rarely - deterioration of liver function; in isolated cases - gum hyperplasia. With long-term use in high doses, dyspeptic symptoms, increased activity of liver transaminases, and intrahepatic cholestasis are possible.
From the central nervous system and peripheral nervous system:
headache. With long-term use in high doses, paresthesia, muscle pain, tremors, mild visual disturbances, and sleep disturbances are possible.
From the hematopoietic system:
in isolated cases - leukopenia, thrombocytopenia.
From the urinary system:
increase in daily diuresis. With long-term use in high doses, renal dysfunction is possible.
From the endocrine system:
in isolated cases - gynecomastia.
Allergic reactions:
skin rash.
Local reactions:
with intravenous administration, a burning sensation at the injection site is possible.
Within 1 minute after intracoronary administration, the negative inotropic effect of nifedipine, an increase in heart rate, and arterial hypotension may occur; these symptoms gradually disappear after 5-15 minutes.
When used simultaneously with antihypertensive drugs, diuretics, phenothiazine derivatives, the antihypertensive effect of nifedipine is enhanced.
When used simultaneously with anticholinergic drugs, memory and attention problems may occur in elderly patients.
When used simultaneously with beta-blockers, severe arterial hypotension may develop; in some cases - the development of heart failure.
When used simultaneously with nitrates, the antianginal effect of nifedipine is enhanced.
When used simultaneously with calcium preparations, the effectiveness of nifedipine decreases due to an antagonistic interaction caused by an increase in the concentration of calcium ions in the extracellular fluid.
Cases of the development of muscle weakness have been described when used simultaneously with magnesium salts.
When used simultaneously with digoxin, it is possible to slow down the excretion of digoxin from the body and, consequently, increase its concentration in the blood plasma.
When used simultaneously with diltiazem, the antihypertensive effect is enhanced.
When used simultaneously with theophylline, changes in the concentration of theophylline in the blood plasma are possible.
Rifampin induces the activity of liver enzymes, accelerating the metabolism of nifedipine, which leads to a decrease in its effectiveness.
When used simultaneously with phenobarbital, phenytoin, carbamazepine, the concentration of nifedipine in the blood plasma decreases.
There are reports of an increase in the concentration of nifedipine in the blood plasma and an increase in its AUC when used simultaneously with fluconazole and itraconazole.
When used simultaneously with fluoxetine, the side effects of nifedipine may increase.
In some cases, when used simultaneously with quinidine, a decrease in the concentration of quinidine in the blood plasma is possible, and when nifedipine is discontinued, a significant increase in the concentration of quinidine is possible, which is accompanied by a prolongation of the QT interval on the ECG.
Cimetidine and, to a lesser extent, ranitidine, increase the concentration of nifedipine in the blood plasma and, thus, enhance its antihypertensive effect.
Ethanol may enhance the effect of nifedipine (excessive hypotension), which causes dizziness and other undesirable reactions.
Nifedipine should be used only in a clinical setting under the strict supervision of a physician for acute myocardial infarction, severe cerebrovascular accidents, diabetes mellitus, liver and kidney dysfunction, malignant arterial hypertension and hypovolemia, as well as in patients on hemodialysis. In patients with impaired liver and/or kidney function, the use of nifedipine in high doses should be avoided. Elderly patients are more likely to have decreased cerebral blood flow due to acute peripheral vasodilation.
When taken orally, nifedipine can be chewed to accelerate the effect.
If chest pain occurs during treatment, nifedipine should be discontinued. Nifedipine should be discontinued gradually, since withdrawal syndrome may develop if it is suddenly stopped (especially after long-term treatment).
When administered intracoronarily in the presence of stenosis of two vessels, nifedipine cannot be administered into the third open vessel due to the danger of a pronounced negative inotropic effect.
During the course of treatment, avoid drinking alcohol due to the risk of excessive reduction in blood pressure.
Impact on the ability to drive vehicles and operate machinery
At the beginning of treatment, you should avoid driving vehicles and other potentially dangerous activities that require rapid psychomotor reactions. In the process of further treatment, the degree of restrictions is determined depending on the individual tolerance of nifedipine.
Adequate and strictly controlled studies of the safety of nifedipine during pregnancy have not been conducted. The use of nifedipine during pregnancy is not recommended.
Since nifedipine is excreted in breast milk, its use should be avoided during lactation or breastfeeding should be discontinued during treatment.
In experimental studies
embryotoxic, fetotoxic and teratogenic effects of nifedipine were revealed.
Overdose
Symptoms:
headache, facial skin flushing, decreased blood pressure, suppression of sinus node activity, bradycardia, arrhythmia.
Treatment:
gastric lavage with the administration of activated charcoal, symptomatic therapy aimed at stabilizing the activity of the cardiovascular system.
The antidote is calcium; slow intravenous administration of a 10% solution of calcium chloride or calcium gluconate is indicated, followed by switching to a long-term infusion.
With a pronounced decrease in blood pressure, intravenous administration of dopamine or dobutamine. In case of conduction disturbances, the administration of atropine, isoprenaline or the installation of an artificial pacemaker is indicated. With the development of heart failure - intravenous administration of strophanthin. Catecholamines should be used only in case of life-threatening circulatory failure (due to their reduced effectiveness, a high dosage is required, which increases the risk of increasing the tendency to arrhythmia due to intoxication).
It is recommended to control blood glucose and electrolytes (potassium and calcium ions), as insulin release is impaired. Hemodialysis is not effective.
Interaction with other drugs
The severity of the decrease in blood pressure increases with the simultaneous use of other antihypertensive drugs, nitrates, cimetidine (to a lesser extent, ranitidine), inhalational anesthetics and tricyclic antidepressants.
Medicines from the group of blockers of “slow” calcium channels can further enhance the negative inotropic effect (decreased force of heart contraction) of antiarrhythmic drugs such as amiodarone and quinidine.
Nifedipine causes a decrease in the concentration of quinidine in the blood plasma; after discontinuation of nifedipine, a sharp increase in the concentration of quinidine may occur.
Increases the plasma concentration of digoxin and theophylline, and therefore the clinical effect and the content of digoxin and theophylline in the blood plasma should be monitored.
Inducers of microsomal liver enzymes (rifampicin, etc.) reduce the concentration of nifedipine.
In combination with nitrates, tachycardia increases. The hypotensive effect is reduced by sympathomimetics, non-steroidal anti-inflammatory drugs (suppression of Pg synthesis in the kidneys and Na+ and fluid retention in the body), estrogens (fluid retention in the body). Calcium supplements may reduce the effect of slow calcium channel blockers.
Nifedipine can displace drugs characterized by a high degree of binding from protein binding (including indirect anticoagulants - coumarin and indanedione derivatives, anticonvulsants, non-steroidal anti-inflammatory drugs, quinine, salicylates, sulfinpyrazone), as a result of which their concentration in the blood plasma may increase .
Suppresses the metabolism of prazosin and other alpha-blockers, as a result of which the hypotensive effect may be enhanced.
Nifedipine inhibits the elimination of vincristine from the body and may cause increased side effects of vincristine; if necessary, the dose of vincristine is reduced.
Lithium preparations can increase toxic effects (nausea, vomiting, diarrhea, ataxia, tremor, tinnitus).
Procainamide, quinidine, and other drugs known to prolong the QT interval may increase the risk of significant QT prolongation.
Grapefruit juice suppresses the metabolism of nifedipine in the body, and therefore their simultaneous use is contraindicated.
Calcigard retard - official instructions for use
INSTRUCTIONS for medical use of the drug
Registration number:
Trade name of the drug:
International nonproprietary name:
Dosage form:
extended-release film-coated tablets.
Compound
1 tablet contains: Tablet core: active substance: nifedipine 20 mg; excipients: lactose monohydrate 20.00 mg, starch 13.44 mg, microcrystalline cellulose 15.00 mg, polysorbate-80 1.00 mg, macrogol 6000 1.00 mg, stearic acid 1.00 mg, povidone (K-30 ) 6.00 mg, magnesium stearate 1.56 mg, sodium lauryl sulfate 2.00 mg; Film coating: hypromellose (15 cps) 2.66 mg, ethylcellulose 0.40 mg, diethyl phthalate 0.26 mg, titanium dioxide 0.26 mg, chocolate brown varnish 0.40 mg, talc 0.02 mg.
Description : Round, biconvex, brown, film-coated tablets with a notch on one side.
Pharmacotherapeutic group:
blocker of “slow” calcium channels.
ATX code : [C08CA05]
pharmachologic effect
Pharmacodynamics Selective blocker of “slow” calcium channels, a 1,4-dihydropyridine derivative. It has a vasodilating, antianginal and hypotensive effect. Reduces Ca 2+ current in cardiomyocytes and smooth muscle cells of coronary and peripheral arteries; in high doses inhibits the release of Ca 2+ from intracellular stores. Reduces the number of functioning channels without affecting the time of their activation, inactivation and recovery. It uncouples the processes of excitation and contraction in the myocardium, mediated by tropomyosin and troponin, and in vascular smooth muscles, mediated by calmodulin. In therapeutic doses, it normalizes the transmembrane Ca 2+ current, which is disturbed in a number of pathological conditions, primarily in arterial hypertension. Does not affect the tone of the veins. Strengthens coronary blood flow, improves blood supply to ischemic areas of the myocardium without developing the “steal” phenomenon, and activates the functioning of collaterals. By dilating the peripheral arteries, it reduces the total peripheral vascular resistance, myocardial tone, afterload, myocardial oxygen demand and increases the duration of diastolic relaxation of the left ventricle. It has virtually no effect on the sinoatrial and atrioventricular nodes and does not have antiarrhythmic activity. Increases renal blood flow, causes moderate natriuresis. The negative chrono-, dromo- and inotropic effects are overlapped by reflex activation of the sympathoadrenal system and an increase in the number of heart contractions in response to peripheral vasodilation. The onset of effect is 20 minutes when taken orally; duration of effect – 12-24 hours (prolonged “retard” form). Pharmacokinetics Absorption – high (more than 92-98%). Bioavailability – 40-60%. Eating increases bioavailability. Has a “first pass” effect through the liver. Penetrates the blood-brain barrier and is excreted in breast milk. Completely metabolized in the liver. The isoenzymes CYP3A4, CYP3A5 AND CYP3A7 are involved in the metabolism of the drug. The half-life (T1/2) is 3.8-16.9 hours. In patients with liver failure, total clearance decreases and T1/2 increases. There is no cumulative effect. Chronic renal failure, hemodialysis and peritoneal dialysis do not affect pharmacokinetics. With prolonged use, tolerance to the action of the drug develops. Plasmapheresis may enhance elimination.
Indications for use
Coronary heart disease – angina pectoris of exertion and rest (including variant). Arterial hypertension (as monotherapy or in combination with other antihypertensive drugs).
Contraindications
Hypersensitivity to nifedipine or other dihydropyridine derivatives, other components of the drug, cardiogenic shock (risk of myocardial infarction), collapse, severe aortic valve stenosis, chronic heart failure (in the decompensation stage), severe arterial hypotension (systolic blood pressure below 90 mm Hg. Art.), acute period of myocardial infarction (during the first 4 weeks), pregnancy (during the first 20 weeks), lactation period, age up to 18 years (efficacy and safety have not been established).
With caution : severe stenosis of the aortic orifice or mitral valve, hypertrophic obstructive cardiomyopathy, severe bradycardia or tachycardia, sick sinus syndrome, malignant arterial hypertension, mild or moderate arterial hypotension, myocardial infarction with left ventricular failure, unstable angina, simultaneous administration of beta-blockers or cardiac glycosides, simultaneous use of rifampcin, severe cerebrovascular accidents, impaired liver and/or kidney function, hemodialysis (risk of arterial hypotension), old age, gastrointestinal obstruction, pregnancy after 20 weeks.
Pregnancy and lactation
Prescription of nifedipine for pregnant women is indicated only if the expected benefit to the mother outweighs the potential risk to the fetus. Nifedipine is not recommended for use during the first 20 weeks of pregnancy. The drug is excreted in breast milk, so it is recommended to stop breastfeeding while taking the drug. There are no data on the use of Calcigard retard by nursing women.
Directions for use and doses
The dosage regimen is set individually depending on the severity of the disease and the response to the therapy. It is recommended to take the drug during or after meals with a small amount of water. Initial dose: 1 tablet (20 mg) twice a day. If necessary, the dose can be increased. The maximum daily dose of the drug is 120 mg. In elderly patients or patients receiving combination (antianginal or antihypertensive) therapy, with impaired liver function, in patients with severe cerebrovascular accidents, the dose should be reduced.
Side effect
From the cardiovascular system: Manifestations of excessive vasodilation (asymptomatic decrease in blood pressure, development or worsening of heart failure, “flushes” of blood to the facial skin, flushing of the facial skin, feeling of heat), tachycardia, palpitations, arrhythmia, peripheral edema, chest pain cage. Rarely - excessive decrease in blood pressure, fainting; in some patients, especially at the beginning of treatment, angina attacks may occur, which requires discontinuation of the drug; Isolated cases of myocardial infarction have been described. From the central nervous system: Headache, dizziness, increased fatigue, weakness, drowsiness. With long-term oral administration in high doses, paresthesia of the limbs, depression, anxiety, extrapyramidal (parkinsonian) disorders (ataxia, “mask-like” face, shuffling gait, stiffness in the movement of arms and legs, tremor of the hands and fingers, difficulty swallowing). From the digestive system: Dry mouth, increased appetite, dyspepsia (nausea, diarrhea or constipation). Rarely – gum hyperplasia (bleeding, pain, swelling). With long-term use - liver dysfunction (intrahepatic cholestasis, increased activity of liver enzymes). From the hematopoietic organs: Anemia, asymptomatic agranulocytosis, thrombocytopenia, thrombocytopenic purpura, leukopenia. Allergic reactions: Rarely - skin itching, urticaria, exanthema, exfoliative dermatitis, photodermatitis. Very rarely – autoimmune hepatitis. From the musculoskeletal system: arthritis, arthralgia (rarely), swelling of the joints, myalgia, convulsions of the upper and lower extremities. From the urinary system: increased daily diuresis, deterioration of renal function (in patients with renal failure). Other: Rarely – difficulty breathing, cough; very rarely - visual impairment (including transient blindness at the maximum concentration of nifedipine in the blood plasma), gynecomastia (in elderly patients, completely disappearing after discontinuation of the drug), hyperglycemia, galactorrhea, pulmonary edema, bronchospasm, weight gain.
Overdose
Symptoms: headache, facial skin flushing, decreased blood pressure, suppression of sinus node activity, bradycardia, arrhythmia. Treatment: gastric lavage with the administration of activated charcoal, symptomatic therapy aimed at stabilizing the activity of the cardiovascular system. The antidote is calcium; slow intravenous administration of a 10% solution of calcium chloride or calcium gluconate is indicated, followed by switching to a long-term infusion. With a pronounced decrease in blood pressure, intravenous administration of dopamine or dobutamine. In case of conduction disturbances, the administration of atropine, isoprenaline or the installation of an artificial pacemaker is indicated. With the development of heart failure - intravenous administration of strophanthin. Catecholamines should be used only in case of life-threatening circulatory failure (due to their reduced effectiveness, a high dosage is required, which increases the risk of increasing the tendency to arrhythmia due to intoxication). It is recommended to control blood glucose and electrolytes (potassium and calcium ions), as insulin release is impaired. Hemodialysis is not effective.
Interaction with other drugs
The severity of the decrease in blood pressure increases with the simultaneous use of other antihypertensive drugs, nitrates, cimetidine (to a lesser extent, ranitidine), inhalational anesthetics and tricyclic antidepressants. Medicines from the group of blockers of “slow” calcium channels can further enhance the negative inotropic effect (decreased force of heart contraction) of antiarrhythmic drugs such as amiodarone and quinidine. Nifedipine causes a decrease in the concentration of quinidine in the blood plasma; after discontinuation of nifedipine, a sharp increase in the concentration of quinidine may occur. Increases the plasma concentration of digoxin and theophylline, and therefore the clinical effect and the content of digoxin and theophylline in the blood plasma should be monitored. Inducers of microsomal liver enzymes (rifampicin, etc.) reduce the concentration of nifedipine. In combination with nitrates, tachycardia increases. The hypotensive effect is reduced by sympathomimetics, non-steroidal anti-inflammatory drugs (suppression of Pg synthesis in the kidneys and retention of Na + and fluid in the body), estrogens (fluid retention in the body). Calcium supplements may reduce the effect of slow calcium channel blockers. Nifedipine can displace drugs characterized by a high degree of binding from protein binding (including indirect anticoagulants - coumarin and indanedione derivatives, anticonvulsants, non-steroidal anti-inflammatory drugs, quinine, salicylates, sulfinpyrazone), as a result of which their concentration in the blood plasma may increase . Suppresses the metabolism of prazosin and other alpha-blockers, as a result of which the hypotensive effect may be enhanced. Nifedipine inhibits the elimination of vincristine from the body and may cause increased side effects of vincristine; if necessary, the dose of vincristine is reduced. Lithium preparations can increase toxic effects (nausea, vomiting, diarrhea, ataxia, tremor, tinnitus). Procainamide, quinidine, and other drugs known to prolong the QT interval may increase the risk of significant QT prolongation. Grapefruit juice suppresses the metabolism of nifedipine in the body, and therefore their simultaneous use is contraindicated.
special instructions
During the treatment period it is necessary to refrain from taking ethanol. It is recommended to stop treatment with the drug gradually. It should be borne in mind that angina pectoris may occur at the beginning of treatment, especially after recent abrupt withdrawal of beta-blockers (the latter should be withdrawn gradually). The simultaneous administration of beta-blockers must be carried out under conditions of careful medical supervision, as this may cause an excessive decrease in blood pressure, and in some cases, aggravation of symptoms of heart failure. In case of severe heart failure, the drug is dosed with great caution. The diagnostic criteria for prescribing the drug for vasospastic angina are: the classic clinical picture, accompanied by an increase in the ST segment, the occurrence of ergonovine-induced angina or coronary artery spasm, the detection of coronary spasm during angiography or the identification of an angiospastic component without confirmation (for example, with a different voltage threshold or with unstable angina, when electrocardiogram data indicate transient vasospasm). For patients with severe obstructive cardiomyopathy, there is a risk of increased frequency, severity and duration of angina attacks after taking nifedipine; in this case, discontinuation of the drug is necessary. In patients on hemodialysis with high blood pressure, irreversible kidney failure, and a reduced total blood volume, the drug should be used with caution; a sharp drop in blood pressure may occur. Patients with impaired liver function are closely monitored and, if necessary, reduce the dose of the drug and/or use other dosage forms of nifedipine. If during therapy the patient requires surgery under general anesthesia, it is necessary to inform the anesthesiologist about the nature of the therapy being performed. During treatment, positive results are possible with direct Coombs test and laboratory tests for antinuclear antibodies. Caution should be used when co-administering disopyramide and flecainamide due to a possible increase in inotropic effect.
Impact on the ability to drive a car and other mechanisms
In some patients, especially at the beginning of treatment, the drug may cause dizziness, which reduces the ability to drive a car or use other machinery. In the future, the degree of restrictions is determined depending on the individual tolerability of the drug.
Release form
Extended-release film-coated tablets 20 mg. 10 tablets in a blister made of PVC film and aluminum foil. 3, 6 or 10 blisters of 10 tablets with instructions for use in a cardboard box.
special instructions
During the treatment period it is necessary to refrain from taking ethanol.
It is recommended to stop treatment with the drug gradually.
It should be borne in mind that angina pectoris may occur at the beginning of treatment, especially after recent abrupt withdrawal of beta-blockers (the latter should be withdrawn gradually).
The simultaneous administration of beta-blockers must be carried out under conditions of careful medical supervision, as this may cause an excessive decrease in blood pressure, and in some cases, aggravation of symptoms of heart failure.
In case of severe heart failure, the drug is dosed with great caution.
The diagnostic criteria for prescribing the drug for vasospastic angina are: the classic clinical picture, accompanied by an increase in the ST segment, the occurrence of ergonovine-induced angina or coronary artery spasm, the detection of coronary spasm during angiography or the identification of an angiospastic component without confirmation (for example, with a different voltage threshold or with unstable angina, when electrocardiogram data indicate transient vasospasm).
For patients with severe obstructive cardiomyopathy, there is a risk of increased frequency, severity and duration of angina attacks after taking nifedipine; in this case, discontinuation of the drug is necessary.
In patients on hemodialysis with high blood pressure, irreversible kidney failure, and a reduced total blood volume, the drug should be used with caution; a sharp drop in blood pressure may occur.
Patients with impaired liver function are closely monitored and, if necessary, reduce the dose of the drug and/or use other dosage forms of nifedipine.
If during therapy the patient requires surgery under general anesthesia, it is necessary to inform the anesthesiologist about the nature of the therapy being performed.
During treatment, positive results are possible with direct Coombs test and laboratory tests for antinuclear antibodies.
Caution should be used when co-administering disopyramide and flecainamide due to a possible increase in inotropic effect.
Impact on the ability to drive a car and other mechanisms
In some patients, especially at the beginning of treatment, the drug may cause dizziness, which reduces the ability to drive a car or use other machinery. In the future, the degree of restrictions is determined depending on the individual tolerability of the drug.