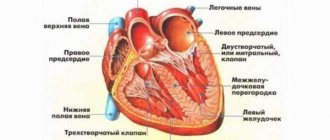
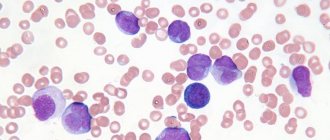
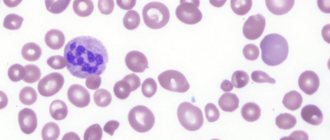
What is paroxysmal nocturnal hemoglobinuria?
Paroxysmal nocturnal hemoglobinuria (abbr. PNH ) is a rare disease in which red blood cells break down prematurely. PNH is an acquired disease of hematopoietic stem cells. Hematopoietic stem cells are created in the bone marrow, the spongy center of the body's long bones. These cells grow and eventually become red blood cells, white blood cells, and platelets. Some hematopoietic stem cells in people with PNH are defective and therefore produce defective blood cells. These defective PNH red blood cells are extremely susceptible to premature destruction by a certain part of a person's own immune system called the complement system.
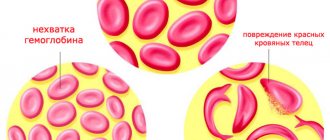
The destruction of red blood cells (hemolysis) by complement leads to the appearance of hemoglobin in the urine (hemoglobinuria). Hemoglobin is a red, iron-rich, oxygen-containing pigment in the blood. People with hemoglobinuria may have dark or bloody urine. This is especially most noticeable in the morning, after the urine has concentrated overnight during sleep. However, hemolysis in people with PNH is an ongoing process (ie it does not occur only at night). Hemoglobin in urine may not always be visible to the eye.
In addition to hemolysis, people with paroxysmal nocturnal hemoglobinuria are also susceptible to developing recurring, potentially life-threatening blood clots (thrombosis). Patients also have some degree of bone marrow dysfunction. Severe bone marrow dysfunction leads to low levels of red and white blood cells and platelets (pancytopenia). The specific symptoms of PNH vary greatly from one person to another, and sufferers usually do not exhibit all the symptoms associated with the disorder.
Classification
Based on the available data on the causes and characteristics of pathological changes, several forms of paroxysmal nocturnal hemoglobinuria are distinguished:
- Subclinical.
- Classic.
- Associated with hematopoiesis disorders.
The subclinical form of the disease is often preceded by aplastic anemia. There are no clinical manifestations of the pathology, but the presence of a small number of defective blood cells is detected only during laboratory tests.
Clinic of nocturnal paroxysmal hemoglobinuria
On a note. There is an opinion that PNH is a more complex disease, the first stage of which is aplastic anemia.
The classic form occurs with typical symptoms; populations of defective red blood cells, platelets and some types of leukocytes are present in the patient’s blood. Laboratory research methods confirm intravascular destruction of pathologically altered cells; hematopoiesis disorders are not detected.
After suffering from diseases leading to hematopoietic insufficiency, a third form of pathology develops. A pronounced clinical picture and intravascular lysis of red blood cells develop against the background of bone marrow lesions.
There is an alternative classification, according to which there are:
- Actually PNH, idiopathic.
- Developing as a concomitant syndrome with other pathologies.
- Developing as a consequence of bone marrow hypoplasia.
Clinic of nocturnal paroxysmal hemoglobinuria. Part 2
The severity of the disease in different cases is not always related to the number of defective red blood cells. Both subclinical cases with a content of modified cells approaching 90%, and extremely severe cases with replacement of 10% of the normal population, have been described.
Signs and symptoms
Symptoms of paroxysmal nocturnal hemoglobinuria occur due to the formation of defective blood cells and because the bone marrow does not produce enough blood cells. The specific symptoms and progression of the disorder vary greatly from one person to another. Some people may have mild symptoms that remain stable for many years; others may have severe symptoms that can progress and cause life-threatening complications.
It is important to note that patients may not have all of the symptoms described below. Affected individuals should talk to their doctor about their specific case, associated symptoms, and overall prognosis.
Premature destruction of red blood cells (hemolysis) is the primary clinical sign associated with PNH. Hemolysis can lead to the formation of hemoglobin in the urine, although many people with hemolysis have no visible hemoglobin in their urine. When hemolysis occurs, the outer wall (membrane) of the red blood cell breaks down (lysis) releasing hemoglobin. Hemoglobin is excreted from the body in urine, causing the urine to become dark or bloody in color (hemoglobinuria). Hemolysis continues and may worsen (for example, a person may have a hemolytic episode) during periods of infection, injury, or stress. Premature destruction of red blood cells can lead to low levels of circulating red blood cells (hemolytic anemia), which is exacerbated by underlying bone marrow dysfunction.
Chronic hemolysis is central to all symptoms and physical manifestations associated with PNH. Mild hemolysis can cause:
- fatigue;
- rapid heartbeat (tachycardia);
- headache;
- chest pain;
- difficulty breathing when doing physical exercise.
If hemolysis is severe, additional symptoms may develop, including fatigue, daytime sleepiness, difficulty swallowing (dysphagia), painful contractions that affect the abdomen, esophagus (esophageal spasms), and in men can cause erectile dysfunction and impotence. Chronic hemolysis can also lead to blood clots, and some patients can develop acute and chronic kidney disease.
About 15 to 30 percent of people with paroxysmal nocturnal hemoglobinuria develop blood clots, especially in the veins (venous thrombosis). The exact cause of blood clots in people with PNH is not fully understood. In addition to red blood cells, defective hematopoietic stem cells can also produce defective platelets. Some researchers believe that these defective platelets are prone to forming blood clots. Chronic hemolysis can also contribute to the formation of thrombi (blood clots).
Blood clots can travel through the bloodstream to various parts of the body, which can lead to life-threatening complications. Blood clots can reduce or cut off blood flow to various organs, especially the stomach, liver, and brain. The specific symptoms associated with venous thrombosis depend on the specific area of the body affected. For example, blood clots affecting the liver can cause:
- jaundice;
- abdominal pain;
- Budd-Chiari syndrome.
Blood clots affecting the stomach and intestines can also cause severe abdominal pain, bloating, or a feeling of fullness. Blood clots affecting the veins of the brain cause symptoms such as headaches or trouble thinking. Blood clots in the lungs can cause shortness of breath, difficulty breathing, and a rapid heartbeat. In rare cases, blood clots can form in the arteries. Blood clots can potentially cause life-threatening complications by cutting off blood flow to vital organs.
All patients with PNH have some degree of bone marrow dysfunction. Individuals with mild bone marrow dysfunction may have no symptoms or only mild symptoms. People with severe bone marrow dysfunction may have low levels of red and white blood cells and platelets (pancytopenia). Red blood cells carry oxygen to the body, white blood cells help fight infections, and platelets allow the body to form clots to stop bleeding. Low levels of circulating red blood cells (RBCs) are called anemia. A low level of white blood cells (leukocytes) is called leukopenia, and a low level of platelets is called thrombocytopenia.
Clinical picture
Marchiafava-Miceli disease most often occurs among young people. In the clinical picture, signs of a hemolytic crisis come to the fore, which can occur after provoking factors - increased physical activity, infections, vaccinations. There is evidence of the possibility of developing a hemolytic crisis when taking ascorbic acid and iron supplements.
Patients often complain of weakness, paroxysmal pain in the lumbar region or abdomen, and headache.
The skin of patients with Marchiafava-Micheli disease is pale and icteric; in some patients, hepato- and splenomegaly appears over time.
The disease received its name because of the peculiarity in which hemoglobinuria, manifested by darkening of the urine, sometimes even turning it black, appears only at night and in the early morning hours. During the day, all subsequent portions of urine become light-colored.
Complications of the disease may include thrombosis of the hepatic veins, inferior vena cava, damage to the mesenteric vessels and vessels of the portal system.
Patients with Marchiafava-Micheli disease often experience bleeding, either due to thrombocytopenia or due to damage to varices. Most patients suffer from severe renal dysfunction, 10% die from infectious complications.
Causes and risk factors
For the development of PNH, two factors are necessary:
- an acquired somatic mutation of the PIGA gene that affects one or more hematopoietic stem cells, creating defective "PNH" blood cells,
- and the process that causes these defective stem cells to multiply and proliferate.
Most likely, paroxysmal nocturnal hemoglobinuria occurs against the background of autoimmune bone marrow failure, as in most cases of acquired aplastic anemia. Researchers believe that defective PNH stem cells survive an erroneous attack by the immune system and proliferate while healthy stem cells are destroyed, leading to the development of PNH. The reason why defective cells survive while healthy cells are destroyed is unknown.
A mutation in the PIGA gene is a somatic mutation, meaning it occurs after conception; it is not hereditary and is not passed on to children. This mutation occurs randomly, for no apparent reason (sporadic). In PNH, this mutation occurs in a single hematopoietic stem cell (a clonal disorder), which then multiplies and proliferates. The reason why PNH cells expand and multiply is not fully understood. Scientists believe that other factors, such as secondary gene mutations or immune factors, are necessary for PNH cells to grow and multiply. Therefore, although a PIGA mutation is required for the development of PNH, its presence is not sufficient to cause the disorder. In some cases, this additional factor has been shown to be a second somatic mutation (besides PIGA) that confers a growth advantage on the mutant cell.
The PIGA gene produces a protein that is required for the creation (biosynthesis) of glycosylphosphatidylinositol (GPI) anchors. These anchors allow certain proteins to attach to the cell membrane. These proteins are called GPI-anchored proteins. In cells with a mutation in the PIGA gene, GPI anchors are not formed and, therefore, GPI-anchored proteins cannot attach to cell membranes. Some of these GPI-anchored proteins serve to protect cells from the immune system. Consequently, a deficiency of these surface proteins makes "PNH" blood cells extremely susceptible to destruction by a part of the immune system known as the complement system. PNH red blood cells are particularly susceptible to premature destruction by the complement system.
The complement system is a complex group of proteins that work together to fight infections in the body. These proteins react to bacteria, viruses and other foreign substances in the body. They work with white blood cells to destroy foreign material in the body. In people with PNH, the complement system mistakenly destroys "PNH" blood cells due to the absence of GPI-anchored proteins, which normally protect blood cells from complement system activity.
Affected Populations
PNH is thought to affect men and women in equal numbers, although some studies show a slight female predominance. The prevalence is estimated to be 0.5–1.5 per million people in the general population. The disease has been described in many racial groups and found in all regions of the world. The disorder may occur with greater frequency in people from Southeast Asia or the Far East, who are more likely to have aplastic anemia. The disorder can affect any age group. The average age of diagnosis is 30 years.
Paroxysmal nocturnal hemoglobinuria was first reported in the medical literature in the second half of the 19th century. This disorder was named paroxysmal nocturnal hemoglobinuria due to the mistaken belief that hemolysis and subsequent hemoglobinuria occur only in intermittent episodes (paroxysmal) and with greater frequency at night (at night). However, although hemoglobinuria may occur paroxysmally, hemolysis continues both day and night.
Disorders with similar symptoms
Symptoms of the following diseases may be similar to those of paroxysmal nocturnal hemoglobinuria (PNH). Comparisons may be useful for differential diagnosis.
PNH with acquired aplastic anemia are closely related conditions, while PNH can also occur in association with some forms of myelodysplasia, such as refractory anemia. People with PNH may simultaneously develop aplastic anemia or myelodysplasia. Researchers believe that PNH may occur due to autoimmune bone marrow deficiency, which causes most cases of acquired aplastic anemia and some cases of myelodysplasia.
- Acquired aplastic anemia is a rare disease caused by profound, almost complete bone marrow failure. Bone marrow is a spongy substance that is found in the center of the long bones of the body. The bone marrow produces specialized cells (hematopoietic stem cells) that grow and eventually become red blood cells, white blood cells, and platelets. In acquired aplastic anemia, the almost complete absence of hematopoietic stem cells eventually leads to low levels of red and white blood cells and platelets (pancytopenia). Specific symptoms associated with acquired aplastic anemia may vary, but include fatigue, recurring infections, dizziness, weakness, headaches, and episodes of heavy bleeding. Most cases of acquired aplastic anemia occur from unknown causes (idiopathic), although researchers now believe that most of these cases are the result of the immune system mistakenly targeting the bone marrow (autoimmunity).
- Myelodysplastic syndrome (MDS, myelodysplasia) is a rare group of blood disorders that result from abnormal development of blood cells in the bone marrow. Three main types of blood cells are affected (i.e., red blood cells, white blood cells, and platelets). Red blood cells (RBCs) carry oxygen to the body, white blood cells (WBCs) help fight infections, and platelets help with clotting to stop blood loss. These abnormally developed blood cells cannot develop normally and enter the bloodstream. As a result, people with MDS have abnormally low levels of blood cells (low blood counts). Common symptoms associated with MDS include fatigue, dizziness, weakness, bruising and bleeding, frequent infections and headaches. In some cases, MDS can progress to life-threatening bone marrow failure or develop into acute leukemia. The exact cause of MDS is unknown, but in about 90 percent of patients, acquired (somatic) genetic abnormalities can be detected in the bone marrow cells. There are no specific environmental risk factors.
- Rarely, people with paroxysmal nocturnal hemoglobinuria may develop leukemia , which is a form of cancer that affects the bone marrow and bloodstream. It is characterized by an uncontrolled accumulation of immature blood cells. Acute forms of leukemia may result in low levels of red blood cells, white blood cells, and platelets (pancytopenia) or high white blood cell counts (leukocytosis) with low levels of red blood cells and platelets.
- Paroxysmal cold hemoglobinuria is a rare autoimmune hemolytic disease characterized by the premature destruction of healthy red blood cells (hemolysis) minutes to hours after exposure to cold. Autoimmune diseases occur when the body's natural defenses against invading organisms mistakenly destroy healthy tissue for unknown reasons. Typically, the lifespan of red blood cells is about 120 days. In a person suffering from paroxysmal cold hemoglobinuria, red blood cells are destroyed prematurely and suddenly by an antibody-mediated process when exposed to temperatures of 10 to 15 degrees Celsius and below.
Surgical treatment
The only way to achieve a cure for Marchiafava-Micheli disease is stem cell allotransplantation, which is carried out according to the results of HLA typing in order to select a suitable donor. Such operations are resorted to very rarely, since they are associated with a large number of complications incompatible with life (veno-occlusive liver disease, graft-versus-host disease). Surgical intervention is recommended for resistance to traditional methods of therapy.
Diagnostics
The diagnosis of paroxysmal nocturnal hemoglobinuria may be suspected in people who have symptoms of intravascular hemolysis (eg, hemoglobinuria, abnormally high serum LDH concentration) without a known cause. Diagnosis can be made based on a thorough clinical assessment, a detailed patient history, and various specialized tests. The main diagnostic test for people suspected of having PNH is flow cytometry, a blood test that can identify PNH cells (blood cells that lack GPI-linked proteins).
Conservative therapy
There are no effective conservative methods that completely eliminate the manifestations of the disease. Therapeutic measures are taken to maintain the patient’s normal condition, reduce the intensity of hemolysis, and the likelihood of complications (renal failure, thrombosis). Conservative therapy for paroxysmal nocturnal hemoglobinuria (PNH) includes the following areas:
- Targeted treatment. The main drug, which is highly effective and affects the main link in the pathogenesis of the disease, is eculizumab. It is a monoclonal antibody that is able to bind to the C5 component of complement and block its cleavage into C5a and C5b. As a result, the formation of proinflammatory cytokines and the membrane attack complex, which cause hemolysis, platelet aggregation and destruction of neutrophils, is suppressed.
- Symptomatic treatment. To enhance the stability of red blood cells, folic acid and vitamin B12 are prescribed; in case of iron deficiency, oral forms of iron medications with vitamin C are prescribed. Anticoagulants are prescribed for the treatment and prevention of thrombosis.
- Immunosuppressive treatment of Marchiafava–Micheli disease. In order to restore and stabilize hematopoietic processes, cytostatics (cyclosporine, cyclophosphamide) are used. In a small number of patients, the use of antithymocyte globulin is effective.
- Other ways to eliminate hemolysis. To stop hemolysis, glucocorticosteroids and androgens are also used, but the effectiveness of these medications is very low. Whole blood transfusions may be used as the primary treatment.
Standard Treatments
Treatment for paroxysmal nocturnal hemoglobinuria targets the specific symptoms present in each person and includes many different therapeutic options.
In 2007, the US Food and Drug Administration (FDA) approved the orphan drug Soliris (eculizumab) for the treatment of PNH. This is the first drug approved to treat this disorder. Soliris does not cure PNH, but it does stop the breakdown of red blood cells and may reduce the risk of thrombosis and improve overall quality of life. Soliris works by blocking the body's complement system, which inadvertently destroys PNH red blood cells. Because Soliris blocks part of the body's natural immune system, it increases the risk of meningococcal infections. Therefore, patients should be vaccinated with meningococcal vaccine at least two weeks before receiving the first dose of Soliris.
In 2020, the FDA approved Ultomiris (ravulizumab) for the treatment of PNH hemolysis. Ravulizumab acts similarly to eculizumab and has been shown to be clinically noninferior to eculizumab. Ravulizumab is given every eight weeks and eculizumab every two weeks.
Additional treatment for PNH is symptomatic and supportive and varies depending on the patient's age, general health, presence of comorbidities, severity of PNH, and degree of bone marrow failure.
Some people with PNH take folic acid (vitamin B9) supplements to ensure they have enough folic acid as the need for folic acid increases when the bone marrow tries to compensate for the hemolytic anemia of PNH by increasing the production of red blood cells (erythropoiesis) in the bone marrow. Additionally, people with iron deficiency, which can occur due to the destruction of red blood cells and subsequent loss of iron in the urine, should be given iron supplements.
Some doctors suggest that people with symptoms of hemolysis should be treated with steroids such as prednisone because such treatment is thought to slow the rate of destruction of red blood cells. However, treatment with steroids such as prednisone is controversial because steroid therapy is not beneficial for everyone and can cause serious side effects, especially if therapy is continued for a long time.
Laboratory diagnostics
The diagnosis of Marchiafava-Micheli disease is made after a thorough examination in hematology centers that have the ability to conduct specific tests and analyses.
In peripheral blood are found:
- erythropenia, leukopenia, thrombocytopenia (the state of inhibition of the general growth of blood cells is called pancytopenia);
- reticulocytosis;
- increase in plasma hemoglobin level;
- decreased iron and folate levels.
Bone marrow examination reveals:
- signs of activation of erythropoiesis (production of red blood cells) due to the accumulation of precursor cells (normoblasts, plasma and mast cells);
- decreased number of granulocytes and megakaryocytes;
- areas of hemorrhage, accumulation of hemolyzed red blood cells in the sinuses;
- at the stage of suppression of hematopoiesis, zones of fatty degeneration and devastation are visible.
Specific tests based on the increased sensitivity of defective erythrocytes to complement under conditions that are most favorable in terms of the composition of the medium are the Hem (acidic) and Hartmann (with sucrose) tests.
Both tests test the "survival" of red blood cells in a blood sample placed in a weak solution. Hem's test is positive when the destruction is 5% or more, and Hartman's is 4% or more.
The Coombs test is performed to exclude a connection with the autoimmune mechanism of cell destruction; it is negative for nocturnal hemoglobinuria.
The coloring of urine indicates a significant content of oxyhemoglobin in it.
A urine test showed that one of the initial signs of nocturnal hemoglobinuria is morning and night urine, colored dark red. Over time, the collected urine separates into layers:
- the liquid on top is transparent, but retains color;
- particles of dead cells of organic origin are determined from below.