A huge number of laboratory tests have now been developed, on the basis of which the condition of the body is assessed, the disease is diagnosed, or the effectiveness of treatment is determined. Urine pH (acidity) is one of the indicators that characterizes the function of the urinary system and indicates the presence of pathology.
When diagnosing gout or uric acid diathesis, urine acidity plays a key role. Determination of urine pH is a standard screening test performed during a medical examination or upon admission to a hospital inpatient department.
This test is part of a general urine test, which, in addition to the acidity level of urine, takes into account the quantity, color, density, presence of cellular elements, proteins and salt crystals.
What is urine acidity
During metabolism, many chemical reactions occur in the human body that are necessary for growth, development and maintenance of life.
For all these reactions to occur, a certain acid-base state must be maintained in the circulatory system and intracellular space.
This is done through various biochemical buffer systems and the release of metabolic products into the environment. The organs responsible for the disposal of by-products include the liver, lungs, skin and kidneys.
The kidneys are the most important excretory organ, because the urine they produce contains nitrogen-containing compounds. These substances, accumulating in the body, can have a detrimental effect on the brain, heart and other vital organs.
In addition, urine is an excellent indicator indicating many changes occurring in the body.
The kidneys have a functional unit called the nephron, which produces urine through ultrafiltration of blood. What is acidity?
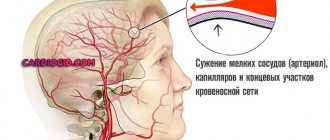
If we perceive urine as an inorganic solution, then it will contain a huge amount of salts, acids, alkalis and free ions that got there during filtration in the renal nephrons. The pH of urine depends on the number of unbonded hydrogen atoms.
With an increase in free H+, the acidic properties of urine will be more pronounced. This means that the higher the level of hydrogen ions in the urine, the more acidic it will be.
The influence of pH on food quality
pH is an important parameter because it affects nutritional characteristics such as texture, taste, aroma and more. Cheese is an excellent example of how pH affects the chemical and physical properties of food. So, as the pH decreases, the protein compounds in this product change and casein loses its ability to interact with water. This results in a firmer cheese consistency, characteristic of aged cheddars and white mold cheeses.
The casein matrix of cheese is created through protein binding. In varieties with an initial pH above 5.0, calcium phosphate crosslinks and casein interacts with water most intensely, creating an elastic, smoother texture, characteristic of young Swiss and cheddar cheeses.
pH standards
Normal acidity is a rather broad term that does not give a complete picture of the state of the body at the moment due to the influence of numerous factors.
There are generally accepted indicators, going beyond which is characterized as the presence of pathology. For urine, the pH will range from 5.0 to 7.0. Short-term fluctuations in acidity from 4.5 to 8.0 can be considered normal if they are short-term and there are no alarming symptoms such as polyuria, oliguria or pain when urinating.
Also, pH values fluctuate depending on the time of day, degree of physical activity, individual characteristics of the body or diet. For example, in the morning the pH is 6-6.5, and in the evening the acidity rises to 7. In addition, the ratio of the liquid secreted to the liquid drunk is of great importance.
Optimal acidity levels in men may be higher than in women due to a higher percentage of muscle mass, as well as a dietary pattern that involves consuming more meat products. Be that as it may, the optimal generally accepted acidity value for adults is in the range from 6.3 to 6.5.
In women during breastfeeding, this figure can rise to 7.8. As a result of the high level of metabolism for newborn children, the acidity numbers will be completely different. The average baby has a urine pH level of 5.4 to 5.9 units, and for premature babies it is 4.8 to 5.4.
Soil acidity scale
Since acidity represents the presence of hydrogen ions in the structure of the earth, more or less of them determines its level. It is designated pH and has its own scale. A reading of 7.0 is the middle of the scale and means the soil is neutral. Values located to the left of this figure indicate that the acidity of the earth increases with decreasing scale numbers. And, conversely, with their increase, the degree of acidification becomes alkaline.
Optimal acidity level for garden crops
All vegetable plants, fruit bushes and trees are very sensitive to acidic soil, and many of them are unable to develop and bear fruit well in such an environment. Therefore, before planting, it is recommended that experienced specialists check the degree of soil acidity and, if necessary, reduce it by neutralizing it.
So, for example, below is a table with acceptable pH levels for vegetable crops:
| Culture | Optimal pH level |
| Watermelon, strawberries, rhubarb, potatoes, chicory | 5,5- 6,4 |
| Tomatoes, peas, cucumbers, corn, carrots, beans | 5,8-6,4 |
| Asparagus | 6.2 |
| Cabbage, broccoli, beets | 6,2-6,6 |
Reasons for changes in urine acidity
Most metabolic products are excreted from the body through the kidneys, so you need to understand that acidity is due to the influence of many factors.
By and large, acidity is a dynamic value that differs from person to person and even changes from one person to another depending on the food consumed, medications taken, lifestyle or time of day. A change in the pH of urinary sediment can occur towards acidification or towards alkalization.
Acidification
Urine acidification is a condition in which the pH becomes less than 5.0. This may occur due to a change in diet, increased physical activity, or pathology of the urinary system.
There are a huge number of diseases that contribute to changes in the acidity of urine. Basically, the pH drops to 5 in diabetes. An acidic urine reaction occurs under the following conditions:
- metabolic acidosis,
- Diabetes mellitus is characterized by a significant change in the composition of urine, not only in terms of a decrease in acidity, but also in the form of an increase in the amount of glucose,
- fever,
- Gout is a common rheumatological disease, the characteristic symptom of which is the acidic environment of urine. The disease is caused by a violation of purine metabolism, as a result of which a large amount of uric acid begins to accumulate in the body,
- leukemia,
- eating low carbohydrate foods,
- An increase in urine acidity may be caused by drugs that increase diuresis. This means that such medications can only be taken in short courses,
- infectious diseases of the urinary system caused by Escherichia coli or mycobacteria,
- chronic renal failure,
- eating foods high in protein. In addition to meat, acid-increasing foods include white bread, fish and cheese,
- sepsis,
- treatment with ascorbic acid in a dosage of more than 2 g per day significantly increases the pH of urine and also increases the risk of developing urolithiasis,
- pathologies of the digestive system.
When urine acidity is elevated for more than 10 days, this is an important laboratory indicator indicating a metabolic disorder or a decrease in the filtration function of the glomerular apparatus of the kidneys.
Also, a slight decrease in the pH level of urinary sediment occurs in newborns. Acidic urine in a newborn is completely physiological and should not cause concern to parents. As the child gets older, the acidity of the urine will level out.
Alkalinization
Alkalinization of urine is a condition in which the pH level becomes more than 7. Alkalinity in the urine can be detected with regular consumption of lactic acid or plant products, as well as with bacterial and metabolic diseases. The reasons for such deviations may be the following factors:
- chronic bacterial urinary tract infection. Microbes are able to ferment nitrogen-containing compounds to ammonia, which leads to an increase in pH,
- hyperkalemia,
- adrenal hormone insufficiency,
- renal tubular acidosis,
- metabolic and respiratory alkalosis,
- passing bloody urine (hematuria),
- increased levels of phosphate-containing compounds in the urine,
- drinking large amounts of mineral water,
- a diet containing large amounts of plant foods, black bread, milk,
- inflammation of the walls of the urinary tract (cystitis, urethritis),
- postoperative period.
In severe pathological processes, chronic renal failure often occurs, leading to alkalization of urine. This is caused by both congenital (primarily wrinkled kidney, pathology of the renal vessels) and acquired (glomerulonephritis, pyelonephritis, diabetic kidney) causes.
Also, temporary alkalization of urine can be caused by intravenous administration of a solution of buffered soda. It is administered in emergency cases accompanied by significant acidification of the blood (sepsis, liver failure, ketoacidotic coma).
Clinically, an increase in pH levels is manifested by general weakness, diffuse headache, nausea and vomiting.
Acidification
If the levels drop, problems begin with the absorption of minerals. Potassium, sodium, calcium and magnesium are completely eliminated from the body. Research was conducted at the University of California. It turned out that when bones become acidic, they become brittle. At first, the relationship could not be officially explained. Later, scientists confirmed that due to acid, calcium is poorly absorbed, and our body is forced to use reserve reserves.
What else is going on inside us? Oxygen is transferred to tissues less actively. In the future, this leads to the development of hypoxia - oxygen starvation. Cells stop receiving enough energy. The sources are there, but the oxidation reaction cannot occur. Because of this, weight increases. The disease is accompanied by a constant feeling of weakness and depression. Slowly but surely the internal organs are changing. The cardiovascular system suffers more.
If you ignore what is happening, diabetes becomes the next step. Similar symptoms can occur with hormonal imbalance; we write about how to determine it here. Problems with joints and bones appear. The latter are also covered with abnormal growths. Excess acid accumulates in the muscles, causing pain. An advanced violation of the acid-base balance with a downward shift has its own name - acidosis.
Methods for determining urine pH
Determining the level of acidity is not the main diagnostic method, but it may indicate the presence of a particular pathology.
It is necessary to take only fresh urine, because long-term storage changes its physicochemical properties, which gives distorted results of the diagnostic test. Determination of pH in urine analysis is carried out in several ways.
Laboratory analysis allows not only to take into account physical and chemical properties, but also to determine the presence of cellular elements (erythrocytes, leukocytes), proteins, crystals, cylinders, sugar and much more.
The analysis is deciphered by a laboratory doctor, and based on the results, additional research methods may be recommended, allowing, if necessary, to clarify the diagnosis. Laboratory tests are the most accurate, so if you suspect the presence of a disease, it is best to resort to them.
At the moment, you can purchase strips for determining acidity at any pharmacy. These are special indicator tests that take into account several indicators. Such strips greatly simplify the analysis and allow you to carry out the test at home.
You need to understand that a one-time test cannot be reliable for several reasons. Firstly, the results are affected by the products consumed.
For example, if a person has a normal pH of 6.0, then a long-term protein diet reduces this indicator, so it is recommended to avoid eating large amounts of meat before the test. Secondly, tests can be inaccurate, so when you receive a result, it is better to re-test.
Test results should only be interpreted by a doctor. Under no circumstances should you prescribe treatment yourself, otherwise this may aggravate the condition and lead to irreparable consequences. At the same time, for some patients, with the permission of the doctor, periodic measurement of urine acidity at home is sometimes recommended.
Regular urine testing to determine pH levels is recommended for people suffering from urolithiasis. This is done to assess the effectiveness of the therapeutic diet and determine the risk of further progress in the formation of stones.
Rating of the best electronic pH meters for measuring soil acidity for 2020
Without a doubt, the first positions in many ratings of the best devices for determining soil pH levels for 2020 are occupied by universal ones, which include several functions.
ATM – 300 – made in China
A universal meter that includes functions of 4 features:
- acidity;
- luminous flux;
- humidity level;
- temperature conditions.
Characteristics:
| Luminous flux measurement | 9 steps |
| Determination of degree of acidification | 12 levels |
| Humidity level | 5 values |
| Temperature range | -9°C to +50°C (16°F to 122°F) |
| Batteries | 9 V each |
| Options | 12.2 x 6.3 x 3.6 cm |
| Electrode dimensions | length – 20 cm, diameter – 0.5 cm |
| Weight excluding batteries | 70.5 gr. |
The kit includes:
- tester;
- instructions for use in English and Russian;
- package.
Mode of application:
- To use the meter, it must be held by the electrode body.
- Lower it into the ground to such a depth as to ensure tight contact with it.
- Leave for 30-60 seconds. to fully record the results on the screen. Remove the electrode from the soil, press the “OF” button and clean it.
acidity meter ATM – 300
Advantages:
- Versatility;
- Ease of use;
- The device is equipped with an automatic shut-off function that activates after 5 minutes. after the measurement is completed.
Flaws:
- To obtain accurate average values, it is recommended to take several measurements in one area.
Multimonitor Luster Leaf Rapitest 1880 – made in China
This device is equipped with 4 functions and determines:
- pH degree;
- stream of light;
- soil fertility;
- moisture contents.
Characteristics:
| pH measuring range | 3,5-8,0 |
| Measuring span of luminous flux | 0-2000 lux |
| Moisture level measurement interval | 1-10 (10% - 100% RH) |
| Options | 26 x 6 x 3.8 cm |
| Rod length | 19 cm |
| Weight | 122 gr. |
| Project and development | USA |
Multimonitor Luster Leaf Rapitest 1880
Advantages:
- A special feature of this meter is that it shows soil fertility in a combination of potassium, phosphorus and nitrogen necessary for the life of garden and garden crops. If any of these components are missing in the soil, the multimonitor will immediately notify you about it.
- The Luster Leaf Rapitest 1880 multimonitor does not require batteries to operate.
Flaws:
- This device is applicable only for soil measurements. It is prohibited to use the tester in water or its solutions.
Luster Leaf Rapitest 1835 - made in China
The device of this group is necessary to determine:
- degree of soil acidification;
- temperature conditions;
- fertility level.
Characteristics:
| Options | 15.2 x 3.6 x 4.6 cm |
| Case dimensions | 10.8 x 6.4 cm |
| Electrode length | 16.5 cm |
| Weight | 136 gr. |
| Wire length | 8 cm |
| Project and development | USA |
Equipped with an LR44 power supply and 3 batteries, the charge of which is sufficient for 1000-1200 measurements.
Luster Leaf Rapitest 1835
Advantages:
- ease of reading measurement indicators on the display;
- guarantee of accuracy of results.
The Luster Leaf Rapitest 1835 tester was recognized in 2013 as a favorite among similar inventions. An independent jury, as well as experienced gardeners, set it apart from the rest thanks to its innovative technology, exclusivity and ability to perfectly solve gardening problems.
Flaws:
- There are no significant ones.
Soil pH meter 3 in 1 – made in China
This device measures:
- degree of soil acidity;
- humidity level;
- ground illumination.
Characteristics:
| pH level measuring range | 3,5-8,0 |
| Humidity detection interval | 0-10 |
| Luminous flux scale | 0-2000 lux |
| Options | 26 x 6 x 3.7 |
| Rod length | 20 cm |
| Weight | 65 gr. |
| Possible deviations in readings: | |
| pH | 0.5 |
| illumination | 50.0 lux |
| humidity | 0.1 |
The kit includes:
- device;
- additional electrode;
- warranty card;
- package.
Mode of application:
This measuring tool is very easy to use and does not require special knowledge and skills. It can be used by both professionals and beginners.
So, to measure the degree of illumination of the ground, it is enough:
- put the lever in the appropriate position;
- insert the electrode into the soil to the required depth;
- turn the screen of the built-in solar battery towards the light source.
In this case, nothing should interfere with the light reaching the tester.
To measure soil pH levels:
- set the switch to the appropriate function;
- clean the electrode rod with a piece of fine-grained sandpaper for polishing, and then wipe with a cloth;
- prepare a certain amount of soil by removing foreign objects and debris from it (stones, glass, plastic, weeds);
- moisten the ground;
- immerse the electrode in it until the beginning of the body and wait 5 minutes;
- record the resulting value on the screen.
When measuring soil moisture, you must:
- set the desired mode;
- clean the probe of the device from remnants of previous use;
- lower the tip into the ground;
- take screen readings.
Depending on the displayed values, soil moisture is divided into 3 types:
- from 1 to 3 (red area) – the ground is dry or slightly damp;
- from 3 to 8 (green area) – slightly damp or fairly damp;
- from 8 to 10 (blue area) – soil is too moist.
Soil pH meter 3 in 1
Advantages:
- Multifunctionality;
- Easy to use.
Flaws:
- There are no significant ones.
Green Belt 3 in 1 06-091 – made in Russia
This measuring device performs 3 functions:
- luminous flux strength;
- pH level;
- presence of moisture in the soil.
Exploitation:
Before each use, be sure to clean the device rods.
Having set the switch to the required function, immerse the electrodes into the ground until they come into full contact with it. After waiting a certain period of time, record the result shown and compare it with the scale presented in the instructions.
When recharging the meter, its screen should be turned towards a source of sunlight and left until the battery is fully charged.
Green Belt 3 in 1 06-091
Advantages:
- versatility and ease of use;
- efficiency;
- recharging only from a solar battery.
Flaws:
- No significant ones were noted.
Luster Leaf Rapitest 1847 - made in China
The advantage of this pH meter is the programmed base of the required degree of acidity for 4 hundred fruits, vegetables and ornamental plants. This function also allows device owners to independently create their own list of cultivated crops and the ability to save it in the analyzer’s memory.
Characteristics:
| Device parameters | 19.5 x 6.5 x 3.0 cm |
| Case dimensions | 10 x 6 x 3 cm |
| Rod length | 16.5 cm |
| Wire size | 8 cm |
| Weight | 150 gr. |
| Acidity measurement interval | 3.5 – 9.0 pH |
| Project and development | USA |
Method of application: Equipped with a power supply and 3 batteries intended for 1000-1200 measuring procedures.
In order to start measuring, you should select the required plant or crop from the list provided on the screen, next to which the optimal value of the soil pH level is indicated, and then carry out the measurement process. The resulting result will be displayed next to the existing value. By comparing the values, decide on pH adjustment.
Luster Leaf Rapitest 1847
Advantages:
- Availability of your own database with values;
- The ability to supplement the basics with plants grown in a specific area.
Flaws:
- No.
Luster Leaf Rapitest 1845 – made in China
The digital tester has 1 function - measuring soil pH.
Characteristics:
| Device parameters | 26 x 2.5 x 4.5 cm |
| Case size | 11 x 4 x 2.5 cm |
| Electrode length | 14.5 cm |
| Weight | 85 gr. |
| Measuring range | 3.5 – 9.0 pH |
| Interval between divisions | 0.1 pH |
The device is equipped with a power supply and 3 batteries. Their charge is enough to carry out 1000-1200 measurements. In addition to the instructions, a booklet is attached with information on the recommended degree of soil acidity for 400 plants.
Luster Leaf Rapitest 1845
Advantages:
- comfortable in using;
- The values on the display are easy to see.
Flaws:
- Monofunctional.
ETP-330 – made in China
A representative of this brand performs only one function - measuring the degree of soil acidification.
Characteristics:
| Dimension span | 3-10 |
| Possible deviation of the result | 0.5 pH |
| Electrode rod length | 20 cm |
| Project and development | USA |
Included:
- device with 1 electrode,
- Russian-language instructions,
- package.
Operating rules:
- lower the probe rod into the ground until it makes good contact with it;
- after 40-60 seconds. fix the position of the arrow on the screen;
- Determine the acidic, neutral or alkaline soil level by the color of the indicator.
ETP-330
Advantages:
- Ease of use;
- Clarity of the obtained values.
Flaws:
- Only one function.
BIOGROD company Klioma Service - made in China
The device is equipped with one function.
Characteristics:
| Type | Portable, portable |
| Measuring range | 2.0 – 7.0 pH |
| Possible deviations +/- | 0.1 pH |
Mode of application:
- If necessary, clean the electrode tip.
- Remove foreign objects and debris from the soil.
- Moisten the soil.
- Insert the rod vertically to the required depth.
- After waiting for 20-30 seconds, record the readings of the device.
BIOGROD
Advantages:
- ease of use;
- accuracy of readings;
- efficiency;
- no need for power supply.
Flaws:
- Only one function.
Luster Leaf Rapitest 1817 – made in China
A very comfortable, simple and miniature analyzer of pH and moisture levels in the soil is quite popular among gardeners and lovers of indoor flowers. It allows you to effectively take measurements close to the roots of crops without causing any significant harm to them.
Characteristics:
| Measuring range of soil acidification | 4-8 pH |
| Humidity span | ABCD |
| Project and development | USA |
Method of use:
Immerse the electrode vertically into the prepared soil to 2/3 of its length. The distance to the plant stem should be at least ½ the radius of the pot. After a few seconds, you will see two indicators on the display - pH level and soil moisture.
Luster Leaf Rapitest 1817
Advantages:
- This analyzer does not require additional electrical power or batteries, as it is charged using a solar battery;
- It includes a booklet with a list of the optimal degree of acidity for 50 crops and humidity for 100 plant species.
Flaws:
- There are no significant ones.
Luster Leaf Rapitest 1810 – made in China
This miniature soil acidification analyzer is very easy to use. With its help, you can take pH measurements, both in pots of indoor flowers, in flower beds and in garden plots.
Measurements of values should be carried out similarly to other devices, but, according to the recommendations of experts, it is advisable to do this procedure at some distance from the plant, and not near the root system itself.
Important! This device is not suitable for measuring values of water and solutions.
To extend the life of the pH meter, after each measurement process, it must be rinsed with water and wiped with a napkin.
Luster Leaf Rapitest 1810
Advantages:
- the rod is made of stainless steel;
- does not require electrical power;
- In addition to the instructions, it is equipped with a guide to the required pH level for 50 plants.
Flaws:
- Only for soil, cannot be measured in liquids.
pH meter calibration
When calibrating a pH electrode, it is important to select buffers that “bracket” the expected pH of the sample being tested. Bracketing is just such a process for calibrating a pH meter, as it is done at points above and below the expected value.
The calibration process is quite simple. When a pH sensor is placed in a solution, the voltage generated is converted into a pH value. The pH buffer solution is a solution of known value, with which the device’s performance is compared.
The bias voltage is typically taken to be ±60 mV for a pH 7.01 buffer solution. Significant changes in this value may indicate that the buffer no longer has the pH value stated on the package or that there is a coating on the pH electrode. Experts recommend that the offset be ±30 mV.
Calibrating the pH electrode to pH rated points of 4.01 or 10.01 is called slope adjustment. This value relative to the offset determines the slope of the line used by the pH meter to correlate the mV and pH of the sample being measured. An electrode with a 100% slope will generate 59.16 mV/pH at a solution temperature of 25 °C. Most pH meters are calibrated to a slope of 85-105% at 25°C. Either way, the pH slope of the electrode must be greater than 90%, otherwise it should be replaced. However, over time, all electrodes will deteriorate. This is usually a gradual process. Any significant change in slope from one calibration to the next is also an indication of contamination of the pH buffer. This is why many manufacturers offer CAL testers to help you determine when the probe is deviating from its ideal offset and tilt. A CAL check meter will display a “clean electrode”, “control buffer” and report on the overall condition of the electrode by monitoring its performance during calibration.
How to lower the pH in your pool
To normalize the acidity in the tank, pH-minus preparations are used. They are produced in powder, granular and liquid form. The products are aimed at reducing carbonate hardness of water, CO2 content and alkali concentration. The main constituent substance is sodium bisulfate.
The drugs must be used in accordance with the dosage table depending on the volume of the artificial reservoir and the pH value. Powder and granules are poured into a skimmer, or diluted in a small amount of water. This solution is added directly to the bowl or pump distribution tank. Liquid agents are also used.
When working with drugs, you must follow safety precautions, wear protective clothing, gloves, and goggles. Do not inhale fumes. If the product gets on the skin or mucous membranes, rinse thoroughly under running water. If the drug enters the body, consult a doctor immediately.