Macro- and microelements
07/09/201907/09/2019 Chernenko A. L. 1327 Views calcium, macro and microelements
Today we are looking at ionized calcium, what it is, which shows what the difference is with general calcium. Calcium (Ca) is one of the most important elements in the human body. This element takes part in the processes of regulating heart rate, maintaining vascular tone and stabilizing blood pressure, normalizing the permeability of vascular walls, the formation of bone tissue and teeth (calcium is also responsible for the density of bones and teeth), blood clotting processes, ensuring neuromuscular conduction, etc. d.
Normally, calcium contained in human blood serum is presented in three fractions:
- Ca in protein-bound form;
- ionized calcium in the blood (free calcium);
- Ca in complex with low molecular weight anions.
This article discusses ionized calcium, what it is, when a test for ionized calcium levels is performed, reasons for changes in tests, and what to do if calcium in the blood is below normal.
- 1 What is the difference between total and ionized calcium?
- 2 What does ionized calcium indicate?
- 3 When should you take a blood calcium test?
- 4 How to prepare for an ionized calcium test?
- 5 When is ionized calcium elevated?
- 6 When does the level of ionized Ca in the blood decrease?
- 7 What to do if ionized calcium is low, and how to increase it?
Functions in the body
Calcium in the blood is found in 3 types:
- as part of proteins;
- in the composition of salts;
- in the form of free cations.
The dynamic balance between different types of element is regulated by the endocrine and nervous systems. Only the ionized form of calcium is metabolically active.
Ionized calcium in the blood is involved in the following biochemical processes:
- formation and ensuring the constancy of bone tissue structure;
- ensuring nerve and neuromuscular conduction;
- maintaining cardiac activity and vascular tone;
- control of the blood clotting process;
- regulation of hormone production;
- regulation of the tone of the autonomic nervous system;
- participates in the body's immune reactions.
Calcium. Why is it needed in the body?
Calcium as an element was isolated in 1808 by the English chemist Davy. Afterwards, they began to actively study its properties, including in medicine. Calcium is an essential macronutrient necessary to maintain many vital functions of the body. Its mass is 1.5-2% of the weight of the human body. Almost 98% of the element is contained in bone tissue and teeth, the remaining 2% is present in nerve fibers, muscles, and blood.
Ca ions take part in regulating the activities of almost all organs and systems. Calcium is found in the blood in active and inactive forms. The active form is ionized calcium, which makes up 50%, the remaining 50% is inactive calcium lactate, phosphate, carbonate.
The importance of the element for a person:
- Ensuring the strength of bones, teeth, nails.
- Maintaining the normal functioning of the cardiovascular and nervous systems.
- Ensuring muscle contractility.
- Participation in the process of blood clotting.
- Participation in iron metabolism processes.
- Normalization of endocrine function.
- Maintaining the strength of vascular walls.
How and under what conditions is it produced?
Calcium is not produced in the body, but comes from food. Calcium absorption occurs in the upper parts of the small intestine, which is facilitated by lactose, ascorbic acid, and vitamin D. The role of vitamin D in the process of calcium absorption is especially important.
Calcium absorption is prevented by:
- excess phosphorus in food;
- excess fat;
- excess oxalic acid and phytin;
- lack of vitamin D.
Ionized calcium in the blood is maintained at an optimal and stable level only if renal function is normal, when filtration and reabsorption of calcium cations are not impaired. Calcium is deposited in bone trabeculae.
A stable level of calcium in the blood is maintained by the endocrine system:
- when the level of calcium in the blood falls, the production of parathyroid hormone by the parathyroid glands is activated, which increases the level of calcium by removing it from bone depots and reducing excretion of the cation by the kidneys;
- when calcium levels are elevated, the thyroid gland is activated, producing calcitonin, which directs excess calcium into the bone depot and enhances its release.
Hypocalcemia
Low calcium in the body indicates the presence of serious health problems. This condition, known as hypocalcemia, requires careful medical evaluation and treatment.
The causes of hypocalcemia can be various:
- Vitamin D deficiency can lower Ca.
- Development of cancerous tumors.
- Chronic sepsis.
- Sedentary lifestyle.
- Poor nutrition, including diets that limit dairy intake.
- Disturbances in the functioning of the gastrointestinal tract.
- Malfunctions of the liver caused by toxins (alcohol, poisons, etc.).
When calcium is low, the doctor prescribes appropriate treatment. In order to maintain optimal amounts of phosphorus and calcium, the body needs vitamin D. The reduced level should be brought back to normal as quickly as possible to prevent the development of diseases. In some cases, appropriate medications are prescribed. More often than not, nutritional adjustments are sufficient to normalize the indicator.
The diet is replenished with foods high in Ca: hard cheeses (especially Parmesan), almonds, beans, fresh herbs (basil, parsley, etc.), crabs, pistachios, etc.
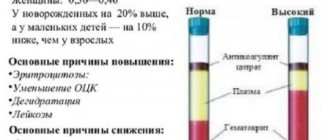
Indicator table is normal
| age | ionized calcium (mmol/l) | total calcium (mmol/l) | ||
| pediatrics | ||||
| Newborns | 1,0-1,30 | 1,90 – 2,60 | ||
| From 3 months to 2 years | 1,10-1,37 | 2,25 – 2,75 | ||
| From 2 to 15 years | 1,10-1,31 | 2,20 – 2,70 | ||
| therapy | ||||
| m | and | m | and | |
| From 15 to 50 years | 1,05-1,26 | 1,10-1,25 | 2,10 – 2,50 | 2,20 – 2,50 |
| Over 50 years | 1,10-1,25 | 1,10-1,25 | 2,20 – 2,50 | 2,20 – 2,50 |
In women and men
From the age of 16 to the age of 60, the microelement content remains virtually unchanged - 2.1 -2.5 mmol/l. Ionized calcium in adults ranges from 1.15-1.29 mmol/l.
Level after 60 years
In older people and in old age, there is a decrease in the concentration of this electrolyte in the blood. Average values are 2-2.45 mmol/l. The doctor determines the individual normal limit during this period based on survey data, examination and the results of other laboratory and instrumental diagnostic methods.
During pregnancy
A decrease in calcium to 2.1 mmol/l is considered a physiological feature during pregnancy and lactation. Typically, this condition does not require correction with medications, since their use can negatively affect the health of the baby and mother.
Watch the video about the role of calcium in the body and its norm:
Symptoms of increase and decrease
The amount of calcium in the blood should be stable, corresponding to normal levels.
Changes in the amount of this macroelement, either in one direction or the other, can have serious consequences for the body.
Hypocalcemia
If calcium levels are moderately reduced, then the clinic is most often absent.
If the decrease in the amount of calcium is pronounced, then neuromuscular excitability increases, leading to:
- to the appearance of cramps and spasms in the muscles of the arms, legs, and back;
- to paresthesia of individual parts of the body;
- to the development of arrhythmias of varying severity.
Progressive hypocalcemia leads to the development of diffuse encephalopathy, which manifests itself:
- depression;
- dementia;
- various psychoses.
In the chronic form of hypocalcemia, patients often note trophic changes in the skin and its appendages:
- dryness, peeling, increased skin vulnerability;
- brittle nails;
- hair loss.
However, if the concentration of calcium cations in the blood is quickly restored, all symptoms gradually disappear. If the amount of calcium in the blood drops sharply, especially its ionized form, a serious condition occurs called tetany or hypocalcemic crisis.
The symptoms of tetany are as follows:
- painful tonic spasms of symmetrical muscle groups;
- labored breathing;
- numbness of the limbs;
- polyuria;
- diarrhea;
- changes in blood pressure;
The main danger of this condition is possible cardiac or respiratory arrest.
Hypercalcemia
With a mild and transient form of hypercalcemia, the clinic may be absent.
For more severe forms, the clinical picture is expressed:
- Central nervous system (lethargy, drowsiness; memory impairment, transient psychoses, possibly coma);
- neurology (muscle weakness, paresthesia, myalgia);
- Gastrointestinal tract (anorexia, constipation, abdominal pain);
- heart and blood vessels (increased blood pressure, tachycardia, blockades and arrhythmias);
- kidneys (increased urine production, retention of nitrogenous metabolites in the body, impairment of all kidney functions).
Lack of calcium in the body symptoms in women
In the blood, the element is presented in two fractions: free or ionized. The second type accounts for approximately half of the total amount in the blood plasma. The second part is associated with plasma albumin and makes up from 40 to 45% of the total mass of the substance in the body, 5–10% is deposited in the form of phosphate, citrate or bicarbonate compounds.
Ionized calcium takes part in all vital processes of the body:
- work of the heart and blood vessels, regulation of heart rhythm,
- conduction of nerve impulses, contraction of smooth and skeletal muscles - under the influence of calcium ions, muscle fibers contract and relax; with a deficiency of the element, the likelihood of spasms and convulsions increases,
- regulation of the functioning of cell walls - calcium ions change the permeability of the cell membrane and take part in the formation of the osmotic pump, through which intracellular metabolism occurs,
- regulation of the blood clotting process - ions release enzymes that fold plasma proteins,
- the work of the exocrine and internal secretion glands,
- metabolism, activity of various hormones and enzymes,
- building bone, muscle tissue, and tooth enamel.
It is necessary that this element in the blood be within physiologically normal values, since the regulation of many processes that are indicators of health depends on this.
A blood test for ionized calcium is carried out together with a blood pH test; the indicators depend on each other: as the pH decreases, the level of the ionized element in the blood increases.
Tests are performed if one of the following symptoms is suspected:
- signs of a decrease or increase in the level of free calcium in the blood plasma,
- suspicion of malignant tumors of various localizations,
- stomach or intestinal ulcer - in this case, the level of the element will decrease due to impaired absorption in the gastrointestinal tract,
- with a decrease in albumin in the blood,
- before operations of any complexity - to prevent internal bleeding,
- with severe muscle hypotonia, dysfunction of smooth muscles, convulsive syndrome,
- for disorders of the kidneys and excretory system: polyuria, kidney or bladder stones, urinary retention or incontinence,
- if there are problems with the thyroid gland,
- for osteoporosis - to track the process.
The analysis is carried out on an empty stomach, since after eating there is a physiological rise in the level of the substance in the blood, which affects the purity of the indicators.
Blood for analysis is taken from a vein on an empty stomach. To obtain an indicator that is closest to physiological norms, you must follow several rules:
- during the day before blood sampling, it is not recommended to eat fried foods, fatty foods, and you should also limit sweets and spices - some foods cause a physiological release of this element into the blood,
- It is contraindicated to drink alcoholic beverages, soda or energy cocktails - it is recommended to drink clean water without gas during the day before the tests,
- Before donating blood, it is recommended to abstain from food for 8–10 hours,
- it is undesirable to perform tests on the biochemical composition of blood immediately after an X-ray, ultrasound examination or physical procedures - this may affect the purity of the indicators,
- Some medications can affect the level of the substance in the blood: diuretics, hormonal agents such as estrogens or androgens, insulin and diabetic drugs, magnesium salts, phosphates, ergocalciferol, vitamins A and D, therefore it is recommended to stop taking medications before the test.
If possible, drug withdrawal should occur one week before blood sampling.
The normal level of a substance in the blood depends on the patient’s age:
- in children of the first year of life the norm is 2.1–2.7 mmol/l,
- in the period from one year to 14 years, the normal value is in the range of 2.2–2.7 mmol/l,
- in patients over 14, the norm for free calcium is 2.2–2.65 mmol/l.
Deviations from the norm indicate the presence of a serious pathology in the body and are accompanied by corresponding symptoms.
If there is a lack of ionized calcium in the blood, symptoms of hypocalcemia will be observed:
- dizziness,
- tension headaches, prolonged migraines,
- increased reflexes, convulsive syndrome,
- thinning and hair loss, dryness and excessive flaking of the skin,
- premature destruction of bones, teeth, articular discs,
- tachycardia,
- bleeding disorders.
With an excess of the substance in the blood, various pathologies will also be observed:
- forced immobility,
- muscle weakness,
- disturbance of consciousness,
- strengthening reflexes,
- nausea, vomiting, general exhaustion and refusal to eat,
- tachycardia.
In advanced cases, vascular calcification, heart and kidney failure develop.
Low or high levels of a substance in the blood indicate disturbances in the functioning of various organs and systems. Hypercalcemia indicates one of the possible pathologies:
- development of acute renal failure,
- hereditary diseases associated with the metabolism and release of substances,
- Williams syndrome or milk-alkali syndrome,
- granulomatous diseases,
- tumors of the bone marrow and other hematopoietic organs,
- adrenal insufficiency,
- the presence of malignant tumors,
- hyperthyroidism, parathyroid hyperplasia, thyrotoxicosis,
- may occur during forced immobility, for example, during immobilization of limbs due to fractures.
While low levels of calcium ions can signal the following diseases:
- hereditary diseases of the parathyroid glands associated with pathology of the X chromosome,
- chronic kidney disease, nephrotic syndrome,
- liver failure, cirrhosis, organic liver damage,
- acute pancreatitis with necrotic degeneration of the pancreas, pancreatic cancer.
The level of free calcium is also affected by the presence of vitamin D3, which is responsible for the absorption of calcium in the body:
- with a lack of vitamin D there will be a decrease in free calcium,
- excess D3 also affects calcium levels (it increases proportionally).
Determination of calcium in the blood is included in the biochemical analysis. In our body, this element is found in two forms - ionized, that is, free, and bound to proteins.
The norm of ionized calcium in the blood is at least 45% of the total indicators of the element. It is the assessment of the free fraction that is most informative for doctors.
Using this study, you can identify a number of diseases, as well as confirm or refute the initial diagnosis.
- Probably, each of us knows from childhood that we need Ca for the growth and strengthening of bones, but this is not its only task.
- Free calcium in our body is involved in many vital processes, namely:
- Responsible for bone growth.
- Participates in the coagulation process.
- Participates in regulating enzyme activity.
- Responsible for the conduction of nerve fibers.
- Plays an important role in muscle contraction, including the heart.
- Participates in the synthesis of hormones.
- Responsible for strengthening blood vessels.
- Participates in the formation of the immune system.
Considering this, it is safe to say that it is very important to control the level of calcium in the blood. The levels of ionized calcium in the blood depend on the age group of the patient.
It is worth noting that these are only average indicators of calcium levels in the blood. For each adult patient and child, doctors calculate individual indicators taking into account many factors.
Norma's age
| Child under 1 year | 1.01-1.38 mmol/l |
| Children under 15 years old | 1.27-1.33 mmol/l |
| Adults | 1.15-1.31 mmol/l |
It is very important to maintain these standards, because not only a lack of Ca is dangerous for human health, but also its excess. It is worth noting that government institutions most often conduct research only to determine total calcium in the blood. If a patient needs a blood test for ionized calcium, it is better to contact a paid laboratory.
If calcium in the blood is elevated, this can have a detrimental effect on a person's health. Hypercalcenemia leads to the fact that the substance begins to settle on the walls of blood vessels, in the kidneys and liver. This can lead to the development of heart failure, urolithiasis and pathological conditions of the liver and biliary tract. There may be several reasons why you have high calcium:
- Increased vitamin D content.
- Oncological diseases.
- Increased levels of growth hormone.
- Chronic interitis.
- Metabolic disease.
- Endocrine diseases.
- Abuse of foods containing calcium.
The following symptoms may raise suspicions that you have increased Ca:
- Chronic nausea and vomiting.
- Feeling thirsty.
- Decreased ability to work.
- Weakness.
- Heart rhythm disturbances, shortness of breath.
- Convulsive syndrome.
Often, elevated readings may be the result of incorrect analysis. Ionized calcium can be affected by prolonged contact of the test material with air. In this case, you need to take a second test. If the second analysis for ionized Ca showed a sharp increase, this should be the reason for a number of additional diagnostic procedures.
Early identification of the causes of deviation can be the key to successful treatment.
High Ca can be reduced only by identifying the causes of the deviation. If this is an unhealthy diet, you need to adjust the menu, but if the cause of hypercalcenemia is the development of pathology, you need to immediately begin treating the underlying disease.
Calcium deficiency may also indicate the development of certain pathologies in the body. Ionized calcium in the blood is reduced in the following diseases:
- Infectious diseases.
- Lack of vitamin D or magnesium.
- Kidney pathologies.
- Diseases of the pancreas.
- Endocrine diseases.
- Gastrointestinal diseases.
- Postoperative period.
Low Ca is often observed in menopausal women after 50 years of age. This is due to hormonal changes in the body. Also, the leaching of Ca from the body can occur while taking diuretics, with the abuse of salty foods; a decrease in calcium in the blood in women can occur during pregnancy and an unbalanced diet.
Symptoms that ionized calcium is greatly reduced include the following conditions:
- Destruction of teeth and nails.
- Dry skin.
- Hair fragility.
- Tachycardia.
- Slow blood clotting.
- Nervous excitability.
- Dizziness.
- Headaches and muscle pain.
- Brittle bones.
Reasons for promotion and demotion
Ionized calcium in the blood is maintained at a constant level by a complex regulatory system.
The reasons leading to calcium metabolism disorders are as follows:
- insufficient or excessive intake of macronutrients from food;
- impaired absorption of cations in the gastrointestinal tract;
- dysfunction of the endocrine glands (thyroid gland, parathyroid glands; adrenal glands);
- impairment of calcium excretion by the kidneys.
Hypocalcemia
The main causes of hypocalcemia are:
- hypoparathyroidism;
- hypovitaminosis D;
- hyperthyroidism;
- various intestinal diseases that impede calcium absorption;
- diseases leading to acholia, which impedes the metabolism of vitamin D
- chronic alkalosis, which reduces the proportion of ionized calcium in the blood;
- lack of magnesium, which inhibits the secretion of parathyroid hormone.
In addition, hypocalcemia can result from:
- pregnancy and lactation;
- starvation;
- taking corticosteroids, antiepileptic drugs (phenytoin, phenobarbital), anti-tuberculosis antibiotics (rifampicin);
- transfusion of large quantities of citrated blood.
Hypercalcemia
The most common causes of hypercalcemia are:
- hyperparathyroidism;
- neoplastic processes;
- granulomatous diseases (granulomas produce substances that activate vitamin D);
- lack of motor activity in case of multiple injuries and severe diseases (osteoclasts are activated, which leads to bone destruction and hypercalcemia);
- medications (in case of overdose of calcium, vitamin D, thiazide diuretics, theophylline).
downgraded: reasons
Hypocalcemia is expressed as a decrease in the concentration of a macroelement in the blood. The analysis shows the pathology well. This disorder is common and is accompanied by the following symptoms:
- pain in muscles and limbs;
- hand tremors;
- deterioration of health;
- dry skin;
- numbness of hands;
- baldness;
- increased fragility of hair and nails;
- memory loss;
- insomnia;
- osteoporosis;
- pain in the lower back.
Deficiency of ionized and bound calcium is often observed in pregnant women. This leads not only to a deterioration in well-being, but also to disturbances in the formation of the fetus. For this reason, characteristic symptoms cannot be ignored. If hypocalcemia is suspected, the expectant mother should be prescribed calcium supplements. In some cases, it is enough to adjust the diet. Hypocalcemia in nursing mothers leads to deterioration of lactation.
A decrease in calcium concentration in the blood occurs not only due to poor nutrition and vitamin D deficiency. The most common causes of hypocalcemia include:
- deficiency of tissue receptors for parathyroid hormone;
- albumin deficiency (with liver cirrhosis);
- treatment with cytostatics;
- acute alkalosis;
- hypoparathyroidism (primary or after surgery).
In newborns, hypocalcemia is often associated with disturbances in calcium metabolism in the mother. Gradually the indicators should level out. For this purpose, vitamins and other medications may be prescribed. In children of the first year of life, a lack of ionized calcium in the blood is associated with vitamin D deficiency and rickets. This is especially common in babies born in winter. Walking outdoors in sunny weather helps replenish vitamin D deficiency, since its production is enhanced by exposure to ultraviolet rays.
Hypocalcemia causes dental problems. The emotional state of a person becomes unstable. People with these disorders often become depressed. Also characterized by too rapid changes in mood.
On a note! Hypocalcemia in children of the first year of life can lead to delayed physical and mental development. These babies have bone deformities. Teeth erupt late and quickly deteriorate.
Indications for the study
Indications for the study of ionized calcium in blood serum are:
- clinical signs of calcium excess or deficiency;
- neoplastic processes;
- kidney diseases (KD, pyelonephritis, nephritis, chronic renal failure);
- cardiovascular pathology (coronary artery disease, hypertension, arrhythmias of various origins);
- neurological diseases (convulsions of various origins, sensory disturbances, muscle weakness);
- pathology of the skeletal system;
- pronounced weight loss;
- mental disorders;
- preoperative preparation.
Monitoring calcium levels is carried out in urgent conditions, when large volumes of water-salt solutions are administered to the patient.
How to determine
The amount of ionized calcium in blood serum can be determined by various methods of chemical analysis:
- colorimetry;
- turbidimetry;
- photometry.
The most economical and accurate method is ionometry. The study is carried out using special electrodes; the ions under study are deposited on the membrane of the electrodes, the amount of which is calculated in the analyzer.
Preparing and conducting analysis
An analysis of calcium levels in blood serum requires preparation and compliance with some simple rules:
- blood for analysis is taken strictly on an empty stomach in the morning;
- a week before the examination, it is necessary to stop taking all medications and dietary supplements; if this cannot be done, then the referral form for the study lists all the medications the patient is receiving, their dosage and duration of use;
- in the morning, before blood sampling, smoking is not recommended;
- the day before the analysis, you should give up alcohol, fatty foods, and avoid overeating;
- on the eve of the study, avoid physical and mental overload.
Blood is taken in a treatment room with a disposable syringe in compliance with all rules of asepsis and antisepsis, in compliance with the following recommendations:
- The patient’s shoulder should not be tightly tightened with a tourniquet;
- material for analysis is taken quickly;
- Serum should be separated from cells as quickly as possible;
- the material must be sent to the laboratory within an hour;
- The material should not be stored in the refrigerator.
Preparing for a blood test for calcium - what factors can distort the results?
To obtain the most accurate test results, patients should adhere to the following recommendations:
- It is best to donate blood for Ca in the morning , between 8 and 12 am on an empty stomach. Strong thirst can be quenched with purified still water.
- During the day before testing, you should not overeat : fried, smoked, salty foods, as well as alcohol should be excluded from the diet.
- You should refrain from physical activity 24 hours before testing. The same goes for stressful situations.
- It is better not to carry out a biochemical blood test for calcium immediately after an ultrasound, fluorography, or intravenous drip
To undergo the testing in question, a referral from the attending physician is required, and it can be completed in a public or private medical institution. In the second case, patients have the opportunity to get acquainted with the results faster.
If during the period of taking the specified test, or 1-2 weeks before taking it, the patient was taking any medications , he must inform his doctor about this. In this case, these same medications will be prescribed in the referral for testing.
The following phenomena can affect the result of biochemical analysis for Ca:
- Pregnancy, lactation, active growth of the child. During this period, structural changes occur in the tissues of the body, which affects the amount of calcium in the blood.
- Treatment with diuretics, hormonal therapy, and taking certain vitamins (A, D) help increase calcium levels.
- Anti-inflammatory, anticonvulsant, antitumor drugs, as well as some antibiotics help reduce the amount of Ca in the human body.
Decoding the results
When interpreting the analysis for the content of ionized calcium in the blood, the gender and age of the patient must be taken into account. A discrepancy between indicators and norms does not always indicate the presence of a disease. Such changes may be a consequence of pregnancy, lactation, overly strict diets, or taking medications.
An analysis for ionized calcium is carried out in conjunction with determining blood pH. There is an inversely proportional relationship between these indicators; the lower the blood pH value, the higher the level of ionized calcium.
Hypocalcemia is a condition in which in adults the level of ionized calcium in the blood is below 1.07 mmol/L, and the level of total calcium is below 1.87 mmol/L. The main cause of hypocalcemia is hypofunction of the parathyroid glands.
Hypercalcemia is a condition in which in adults the level of ionized calcium is more than 1.3-1.5 mmol/l, and the level of total calcium is above 3.0 mmol/l, with normal protein content. There are two main reasons for a persistent increase in calcium in the blood: hyperparathyroidism and neoplastic pathology.
Ionized calcium test
If symptoms of hypocalcemia or hypercalcemia appear, you should consult a doctor. Mandatory indications for donating blood to determine ionized calcium are:
- stomach ulcer;
- suspicion of oncology or diagnosed oncological diseases;
- decrease in albumin in the blood;
- upcoming operations (to determine blood clotting);
- dysfunction of smooth muscles;
- disturbances in the urinary system;
- osteoporosis;
- disorders of the thyroid gland.
When a patient is undergoing intensive therapy with intravenous administration of blood products and glucose-saline solutions, calcium levels are monitored daily or even more often, as indicated. The level of calcium ions can be determined only after donating blood for analysis. Preparation for it involves:
- avoiding alcohol consumption the day before the test;
- refusal of fatty, sweet, high-calorie foods the day before donating blood;
- quit smoking 2 hours before donating blood;
- following a diet 2 days before donating blood (you can’t eat nuts or drink sparkling water).
It is not recommended to donate blood less than 24 hours after ultrasound, radiographic examinations, or physical procedures. All this can affect the purity of the analysis. Blood should be donated strictly on an empty stomach.
The analysis can also be affected by taking medications. For this reason, you should consult your doctor before visiting the treatment room. If possible, you should stop taking medications for a while. Otherwise, you should tell your doctor about all medications you are taking.
When to see a doctor
If symptoms suspicious of a calcium metabolism disorder appear, or if the content of total or ionized calcium is changed in the blood donated for preventive purposes, you should immediately consult a physician.
The doctor, after a physical examination, will prescribe additional examinations:
- biochemical blood test (creatinine, urea, proteins);
- ionogram (calcium, phosphorus, chlorine);
- vitamin D and parathyroid hormone content;
- daily urinary calcium excretion;
- sonography and computed tomography of the parathyroid glands;
- bone densitometry.
After the examination, the patient is sent for treatment to a specialist (endocrinologist, nephrologist, oncologist).
Reduced rate
If a patient’s level of calcium ions is low, the following symptoms indicate this:
| Muscle spasms | Osteoporosis | Dry skin |
| Brittle hair | Dizziness | Headache |
| Rachiocampsis | Nervousness | Bad memory |
| High pressure | Arrhythmia |
With these symptoms, hypocalcemia is diagnosed. The reasons that caused this condition are different in nature:
Vitamin D deficiency;
Extensive burn injuries;
Rickets;
Lack of magnesium in the blood;
The period after surgery;
The intestines absorb calcium poorly;
Metabolic alkalosis (acid-base imbalance).
You should know that such a condition with an indicator of less than 0.7 mmol/l is critical and faces the possibility of death.
How to get it back to normal
Methods for correcting changes in calcium metabolism depend on the underlying disease that caused these disorders, the level of calcium in the blood serum and concomitant pathology.
Hypocalcemia
In this condition, it is necessary not only to replenish the calcium deficiency in the blood, but also to eliminate the cause that caused it.
In severe acute hypocalcemic crisis, calcium salts are administered intravenously. The most commonly used is calcium gluconate (10% calcium gluconate + 5% glucose intravenously). Calcium chloride can cause thrombophlebitis, so it is used less frequently.
With moderately severe hypocalcemia (after removal of the thyroid gland or parathyroid adenoma), taking calcium salts orally is quite sufficient. However, with subtotal parathyroidectomy in the setting of renal failure, hypocalcemia can be very severe.
To prevent such complications, it is necessary to administer calcium salts in large doses intravenously during the postoperative period (1 g/day for 5-10 days) before transferring the patient to oral intake of calcium salts and vitamin D.
In case of a chronic decrease in the level of calcium in the blood caused by hypoparathyroidism or renal failure, calcium preparations are prescribed orally along with vitamin D (calcium gluconate 1000 mg/day for 2 doses, calcium citrate 600 mg/day for 2 doses + Calcitriol 0.25-0.5 mg/day).
In the case of hypocalcemia caused by renal failure, treatment should include restriction of dietary phosphate intake to avoid the development of hyperphosphatemia.
Hypercalcemia
Patients with hypercalcemia of any severity are treated in a hospital (nephrological or endocrinological). Severe forms of hypercalcemia are treated in intensive care units. Treatment is aimed at all parts of calcium metabolism in the body.
Suppression of calcium absorption in the intestines
For these purposes use:
- glucocorticoids (prednisolone 5-10 mg/day once, hydrocortisone 100-200 mg IV);
- antimalarial drugs (chloroquine 250 mg/day 1 time);
- phosphate salts of potassium and sodium (potassium phosphate 0.15 mmol/kg IV drip, sodium phosphate 15-30 mmol/day), phosphates are contraindicated in renal failure.
Increased excretion of calcium through the kidneys
First, an isotonic saline solution (0.9%) is administered intravenously, then loop diuretics (Lasix 20-40 mg/day) are prescribed for forced diuresis.
Suppression of bone destruction
Drugs that suppress osteoclast activity are used here:
- calcitonin 4-8 IU/kg/day for 2 injections;
- bisphosphonates (pamidronic acid 15-30 mg IV drip, zoledronic acid 4-8 mg IV drip).
If all of the above methods are ineffective, and the patient’s general serious condition, hemodialysis is used.
Surgical removal of the parathyroid glands
This is the most effective way to treat hypercalcemia due to increased activity of the parathyroid glands.
Why calcium is not absorbed in the body reasons
Improper functioning of the stomach.
As a result of poor nutrition and bad habits, insufficient production of hydrochloric acid and enzymes occurs in the stomach. Without these components, the body is not able to independently absorb various microelements, including Ca.
Fatty acids, in contact with calcium salts, turn into complex deposits that are not only not absorbed by the body, but are also difficult to remove from it.
Oxalic acid.
By consuming foods containing this substance, a person is unable to absorb calcium in the body. It, interacting with the acid in question, turns into difficultly soluble oxalate salts, which accumulate in the organs, leading to serious consequences.
Vitamin D deficiency.
Vitamin D helps the body absorb calcium. Without this component, Ca is not retained in the body and is excreted from it
Please note that in order for vitamin D to be absorbed, the body must consume fatty acids contained in foods such as fatty fish, eggs and vegetable oils.
When the amount of estrogen (female sex hormone) in a woman’s body decreases, a disturbance in the conductivity of calcium in the tissue occurs. The production of female hormones slows down when the reproductive system, due to age, stops functioning.
Also, oral contraceptives, corticosteroids and pathological processes in the gastrointestinal tract lead to impaired absorption of calcium. To exclude possible pathologies, you should undergo a preventive examination by specialists once a year.
Medications
Treatment of calcium metabolism disorders is a complex task. To prevent the transition of hypocalcemia to hypercalcemia and vice versa, doses of all drugs are selected individually, taking into account the disease that caused the pathology, gender, age, and calcium metabolism parameters.
During the treatment period, blood pH, main indicators of blood biochemistry and ionogram are constantly monitored. The duration of the course of treatment is also determined individually: they try to maintain blood calcium levels at the lower limits of normal for hypocalcemia and at higher limits for hypercalcemia.
Hypocalcemia
Monocomponent calcium preparations:
- Vitacalcin 1000 mg/day for 4 doses;
- Scoralite 750-1000 mg/day for 2-3 doses.
Combination drugs
- Natekal 2 tablets/day for 2 doses;
- Calcium D3 Nycomed 2 tablets/day for 2 doses.
Vitamin and mineral complexes:
- Complivit 2 tablets/day for 2 doses
- Multi Tabs 1 tablet/day for 1 dose.
Vitamin D preparations
- Ergocalciferol 3000 IU/day (40-45 days);
- Cholecalciferol 7500-15000 IU/day.
Hormonal drugs
- Parathyroidin 1.0-2.0 ml every other day
Hypercalcemia
Drugs that prevent bone resorption
- calcitonin 6-8 units/kg every 6-12 hours
- diphosphonates: (sodium etidronate 7.5 mg/kg IV drip; sodium pamidronate 15-45 mg/day; sodium alendronate 40 mg/day);
- potassium and sodium phosphates 1000-1500 mg/day.
Drugs that enhance calcium secretion
- Saline solutions and loop diuretics (0.9% saline or 5% glucose solution 4-8 liters/day + furosemide 20-40 mg every 3-6 hours).
Drugs that inhibit calcium absorption
- Glucocorticoids (prednisolone 30-60 mg/day)
- Cellulose sodium phosphate 15 g/day for 3 doses
Traditional methods
Herbal medicine is used after consultation with the attending physician, as an addition to the main treatment.
Hypocalcemia
Freshly washed leaves and young shoots of nettle are passed through a juicer. After which pure nettle juice is diluted with cold boiled water in a ratio of 1:3, the mixture is boiled for 3 minutes. Take 3 teaspoons/day for 3 doses (3 months).
St. John's wort herb (1 tablespoon) is poured with boiling water (1 glass) and kept in a water bath (15 minutes). Leave for 1 hour. Add 100 grams of honey and 1 tablespoon of lemon juice to the infusion. Drink the infusion 3 tablespoons/day for 3 doses (2 months).
Honey and butter in a 2:1 ratio are mixed and melted in a water bath, lemon juice is added to the cooled mixture. Drink 3 tablespoons/day for 3 doses.
Hypercalcemia
Herbal medicine is unlikely to be able to reduce the concentration of ionized calcium in the blood, but it is quite effective against calcifications on the walls of blood vessels.
1 tablespoon of crushed artichoke leaves is poured into 1 glass of boiling water and left to infuse. After the cooled infusion is drunk. Drink artichoke infusion once a day for 1 month.
10 grams of steelweed, dandelion, and burdock roots are poured with vodka (300 ml). They insist for 1 month. Drink 10 drops/day for 2 doses.
Other methods
Diet therapy
Calcium enters the bloodstream in two ways: with food and from bone depots. This determines the role of diet therapy for calcium metabolism disorders.
Ionized calcium in the blood increases or decreases markedly depending on the patient’s dietary preferences.
For hypocalcemia, it is recommended to increase the menu:
- fermented milk products (yogurt, kefir, cottage cheese, cheese);
- soy products (tofu cheese, soy sauce);
- seafood and canned sea fish (shrimp, sardines in oil, sprat, anchovy);
- vegetables and herbs (celery, cabbage, olives);
- dried fruits (raisins, dried apricots);
- cereals (rice, buckwheat).
In case of hypercalcemia, first of all, it is necessary to exclude or reduce the consumption of calcium-rich foods; in addition, the following are recommended:
- dishes high in phosphorus (chicken, turkey, pork);
- sweets, refined sugar;
- pickles;
- vegetables - potatoes, eggplants, tomatoes.
Ural Federal District
Ultraviolet radiation is electromagnetic waves with a length of 180-390 nm. Exposure to UV radiation leads to the formation of molecules with new chemical properties. When exposed to medium-length UV waves (280-329 nm), the production of vitamin D3, vitamin C and A is stimulated. This is what is used in the treatment of rickets.
Hemodialysis and peritoneal dialysis
For life-threatening hypercalcemia, as well as renal or heart failure, peritoneal dialysis or hemodialysis is used. Methods can quickly reduce calcium levels in the blood. However, a rapid decrease in calcium in the blood can lead to a sharp decrease in blood pressure. Therefore, procedures are carried out with constant monitoring of blood pressure and ECG.
The level of the substance in the blood
In the blood, calcium is contained in a certain balance with other substances. The interpretation of the analysis is immediately clear at first glance. Next to the patient’s indicator, the norm corresponding to a certain age group is indicated. The substance can be designated as Ca or Calcium, calcium ions are indicated as Ca+. The standards for the total indicator and ions are different. The amount of substance is measured in mmol/l. The acceptable limits of Ca and Ca+ are as follows:
| Child's age | Ca in mmol/l | Ca+ in mmol/l |
| Children under one year old | 2,1-2,7 | 1,03-1,37 |
| Children under 16 years old | 2,2-2,7 | 1,29-1,31 |
| From 17 years old | 2,2-2,65 | 1,17-1,29 |
Calcium and phosphorus analysis is deciphered by the attending physician, taking into account all factors that may affect the amount of Ca in the patient’s blood. Item content designations may vary depending on the equipment used. The presented norms reflect the average indicators characteristic of a healthy person. The concentration of the substance may be affected by pregnancy or taking medications. Calcium levels can increase when taking vitamins A and D, lithium, progesterone, and diuretics. Insulin, tetracycline, estrogen, glucose, magnesium, albutrol, etc. help reduce calcium in the body.
Possible complications
Disorders of calcium metabolism without proper therapy can lead to very serious consequences.
A lack of ionized, active calcium in the blood serum can lead to such severe pathologies as:
- heart failure;
- blood clotting disorder;
- osteoporosis;
- impaired immunity;
- eye disorders (cataracts, lens dysfunction);
- neurological and mental disorders.
A very serious complication is tetany, in which death occurs when breathing or heartbeat stops.
Hypercalcemia can also lead to serious consequences:
- osteoporosis, pathological fractures;
- ICD;
- acute pancreatitis;
- intestinal obstruction;
- calcific uremic arteriolopathy;
- severe forms of arrhythmia.
The most dangerous condition, the symptom of which is an excess of ionized calcium in the blood, is considered a hypercalcemic crisis, in which death occurs from renal or heart failure.
Ionized calcium in the blood is increased: reasons
An increase in calcium levels in the blood is called hypercalcemia. This pathology does not pass without symptoms. It is accompanied by the following symptoms:
- decreased physical activity;
- increased fatigue;
- heart rhythm disturbances, increased heart rate;
- thirst;
- convulsions.
From time to time a person may experience nausea and dizziness. Sometimes hypercalcemia is accompanied by vomiting. If the disorder is chronic, calcium gradually accumulates on the walls of blood vessels, in the kidneys and liver. Heart failure may occur.
Hypercalcemia does not occur without cause. Pathology develops against the background of poor nutrition or internal disorders. The most common causes of hypercalcemia include:
- increased calcium production in newborns (Williams syndrome);
- metabolic disorders of hemostasis (acidosis type);
- consuming large amounts of vitamin D and foods with calcium;
- malignant bone tumors;
- acute renal failure;
- benign diseases of the thyroid gland;
- blood diseases;
- insufficient function of the adrenal cortex.
On a note! Hypercalcemia is a chronic disease. If parents have problems with calcium metabolism, they need to be more attentive to the health of their children and regularly carry out all the necessary examinations.