Reticulocyte count on blood smear
Counting reticulocytes in a smear after staining them with special dyes is in practice the most used method for counting the number of reticulocytes. This is due to the fact that the method is simple, quite cheap and does not require special expensive equipment, and therefore can be used in any clinical diagnostic laboratory.
After the loss of the nucleus, red blood cells perceive only acidic dyes, and light microscopy does not reveal any intracellular structures in them that make it possible to classify the formed elements as young or mature species. However, reticulocytes can be found using special staining methods. Supravital staining with brilliantcresyl blue or acridine orange, revealing a basophilic substance - granular or thread-like formations, helps to isolate young forms - reticulocytes - from the total mass (1000 cells) of red blood cells in a smear.
Meanwhile, having in the cytoplasm a mesh substance characteristic of reticulocytes, which determines the name of the cells (substantia reticulofilamentosa), not all of them are “the same from the face”, therefore they are divided into 5 groups, each of which has its own characteristics of the location of the basophilic substance (BS):
- Nuclear reticulocytes, which got their name due to the location of the BV in the form of a corolla or nucleus.
- Tangle-shaped cells - BV resembles clumps or a ball.
- Completely reticular reticulocytes - the basophilic substance is represented by a dense mesh;
- Incompletely reticulated forms - the BV remains as separate threads.
- Pulverized reticulocytes are small grains in cells and are the basophilic substance.
Principle. Supravital staining with dyes that reveal the granular-reticular substance of reticulocytes.
Reagents. You can use one of the following dyes: 1) a saturated solution of brilliant cresyl blue in absolute ethyl alcohol. To prepare absolute alcohol, you need to soak 96% ethanol in several changes of calcined copper sulfate powder. For 100 ml of absolute alcohol take 1.2 g of paint;
2) solution of azure 1 according to P. N. Korikov: azure I - 1 g; ammonium oxalate - 0.4 g; sodium chloride - 0.8 g; 96% ethyl alcohol—10ml; distilled water - 90 ml. The paint solution in a closed bottle is placed in a thermostat at 37 °C for 2–3 days and shaken vigorously periodically. Then cool to room temperature and filter through a paper filter.
https://www.youtube.com/watch?v=https:accounts.google.comServiceLogin
The solution is stored in a dark glass container. If sediment appears, the paint should be filtered again; 3) azur II solution: azur II - 1 g; sodium citrate - 5 g; sodium chloride - 0.4 g; distilled water - 45 ml. The solution is left in a thermostat at 37 °C for 2 days, stirring occasionally. To speed up dissolution, the paint can be heated over low heat for 15–20 minutes, without bringing it to a boil. Cool to room temperature and filter. Store in dark glass containers.
Progress of determination. Painting on glass. A well-washed and degreased glass slide is heated over a burner flame.
Using a glass rod, apply a drop of one of the dyes to the glass and prepare a smear of paint using ground glass. Mark the side of the glass on which the paint stroke is applied with a steklograf. In this form, glass can be prepared for future use and stored in a dry, dark place. Apply a drop of blood to a smear of paint, prepare a thin smear from it and immediately place it in a humid chamber for 3–4 minutes (you can use a Petri dish with rolls of moistened cotton wool, gauze or filter paper placed along the edges). Then the smears are air dried.
In smears prepared in this way, red blood cells are colored yellowish-greenish, and the granular-mesh substance is blue.
In vitro staining. Method 1: before use, prepare a working solution of brilliant cresyl blue in a test tube at the rate of 1 drop of 1% potassium oxalate solution and 4 drops of paint solution (reagent 1). Add 0.04 ml of blood to the paint (two pipettes to the 0.02 mark). The mixture is thoroughly but gently mixed and left for 30 minutes. Mix and prepare thin smears.
Method 2: 0.05 ml of dye solution 3 and 0.2 ml of blood are placed in a test tube. The mixture is thoroughly mixed and left for 20–30 minutes. Mix and prepare thin smears.
Method 3: add 0.3–0.5 ml of paint solution 2 and 5–6 drops of blood into a test tube using a pipette from a Panchenkov apparatus. The test tube is closed with a rubber stopper, the mixture is thoroughly but carefully mixed and left for 1–1.5 hours (reticulocytes stain better with an exposure of 1.5–3 hours). Mix and prepare thin smears.
Reticulocyte count. In smears, red blood cells are colored yellowish-greenish, the granular-mesh substance is blue or bluish-violet.
If you do not have a ready-made eyepiece, you can easily prepare it by unscrewing the 7× eyepiece, inserting a piece of paper with a small square cut out into it, and screwing it on. The number of counted reticulocytes is expressed per 1000 or per 100 red blood cells.
This method requires the following reagents:
- Absolute ethyl alcohol: place calcined anhydrous (white) copper sulfate into a dry container with a ground-in stopper and add 1/4 of the absolute alcohol. The contents are shaken. Absolute alcohol (alcohol that does not contain water) can be obtained by dehydrating the alcohol with previously calcined copper sulfate. Dehydration is stopped when copper sulfate stops turning blue.
- A saturated solution of diamondcresyl blue in absolute alcohol: add 1 g of diamondcresyl blue to 80 ml of absolute alcohol.
- Azur I.
The slides are pre-coated with a saturated solution of diamond-cresyl blue. A freshly released drop of blood is spread onto a glass slide with paint, as when preparing a blood smear. The specimen is immediately placed in a moist chamber for 6-8 minutes, then air-dried and examined microscopically (7X eyepiece, 90X objective with condenser raised).
Reticulocytes stained with brilliant cresyl blue
Instead of diamond cresyl blue, for staining reticulocytes, you can use, according to Magina’s recipe, azure I paint (1 g azure I per 40 ml of absolute alcohol; kept at room temperature for 2 days).
https://www.youtube.com/watch?v=ytaboutru
For this method, Alekseev’s paint is used. 1 g of Azur II paint is poured into a 100 ml flask and a reagent of the following composition prepared in a separate flask is added: 5 g of sodium citrate, 4 g of sodium chloride and 45 ml of distilled water. The contents of the flask are slowly heated on an asbestos grid with constant stirring, without bringing to a boil. Cool and filter. The filtrate is used as a working paint solution.
Alekseev’s paint is added to the leukocyte mixer up to the 1.0 mark, the tip is wiped of paint and placed on the table. A deeper than usual injection is made into the finger. Blood is drawn up to 4/5 of the volume of the expanded part of the mixer, making sure that no air bubbles get into it. The blood is quickly blown out of the capillary part of the mixer onto a cotton swab, and the rest of the contents into the well of the slide.
From the hole, the liquid is again drawn into the mixer. By repeating this manipulation two or three times, the blood is mixed with the reagent. Having collected the liquid into the mixer for the last time, it is left in a horizontal position for a minute to stain reticulocytes and platelets. Then shake the mixer for a minute and release 1-2 drops.
Thin smears are prepared from subsequent drops. The preparations are dried in air, fixed with methyl alcohol and stained with Romanovsky paint diluted with 1-2 drops per 1 ml of water for minutes. The paint is washed off with water, and the smears are dried and microscopically examined. In a smear stained according to Alekseev, the reticulocytes are pink with a blue mesh (see figure), and the platelets are bluish-lilac.
Reticulocytes stained according to Alekseev
Reticulocytes and platelets are counted simultaneously per 1000 red blood cells in the same way as described in the topic “Platelets”. Results for reticulocytes are expressed in promille; platelets are calculated per 1 mm3 of blood (for the calculation, the number of red blood cells counted in a given patient is taken into account).
In the blood of healthy individuals there are 5-10% reticulocytes.
Reticulocytes are young red blood cells with a thin blue retina or granularity in the cytoplasm. These cells characterize the activity of red hematopoiesis.
To determine the reticulocyte count, the method of supravital (intravital) staining is used. Smears of brilliant cresyl blue paint are made on the slides in absolute alcohol, and then a blood smear is made on the paint-coated glass in the usual way and placed in a moist chamber for 3-5 minutes, after which it is dried and microscoped with an immersion lens.
Reticulocytes are cells that characterize the increased production of red blood cells in the bone marrow.
They appear in a large percentage in the peripheral blood with hypochromic anemia (“pernicious anemia”), with hemolytic anemia and other diseases. A reduced content of reticulocytes and their complete disappearance in the peripheral blood are observed during exacerbation of hyperchromic anemia.
To prevent aggregation (sticking together) of blood platelets, a skin puncture is made through a drop of a 14% solution of magnesium sulfate applied to the finger. Blood is mixed with magnesium and thin smears are prepared on glass slides, which are then fixed and stained according to Romanovsky for 2 hours.
Find out the cost of writing such a paper!
Reply within 5 minutes! Without intermediaries!
4) reticulocytes, having a granular-mesh substance in the form of individual threads;
5) reticulocytes containing individual grains.
It should be remembered: normally, according to G. A. Alekseev, almost 80% of reticulocytes belong to groups IV – V.
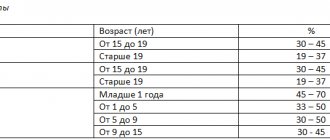
Normally, peripheral blood contains 0.2 - 1% reticulocytes (“%” is the content of reticulocytes from the total number of red blood cells). This indicator reflects the possibility of reticulocytes entering the peripheral blood and their further transformation into mature erythrocytes (maturation), as a variant of the norm, already in the peripheral blood (within several hours).
In normal erythropoiesis, most red blood cells pass through the reticulocyte stage in the bone marrow [3].
Some authors note that women have a slightly higher number of reticulocytes than men. In children in the first days of life, the number of reticulocytes, according to various sources, can reach 5 - 10%, and then decreases.
Reticulocytes are an important indicator of the ability of bone. An increase in their peripheral blood - reticulocytosis, is noted in hemolytic when their number reaches 60% and increases more strongly during crises), in acute (on the 3rd day after blood loss, reticulocyte crisis), polycythemia, in the treatment of iron deficiency anemia, every other day (3 - after the onset of antianemic pernicious anemia, with a lack of oxygen. The presence of the number of reticulocytes allows one to suspect bleeding (for example, patients with abdominal ulcer disease) [5].
that an increase in reticulocytes in the peripheral blood is an expression of good regeneration in the case where there is simultaneously reticulocytosis in the bone marrow, which is called true reticulocytosis. The absence of increased reticulocytes in the brain with an increase in the number in the peripheral blood indicates an increase in bone marrow reticulocytes in the peripheral blood (false reticulocytosis).
Reticulocytosis without a corresponding erythronormoblastic reaction of the brain is observed in irritation of some of its cancerous or inflammatory foci.
- hemolytic anemia of reticulocytes can reach 60% and more, increasing greatly during crises);
- acute blood loss 3 - days after a reticulocyte crisis occurs), in number, an increase in reticulocytes allows hidden bleeding in patients with gastrointestinal typhoid fever);
- malaria;
with polycythemia;
- for iron deficiency anemia several days (3-10) after the start of treatment for pernicious anemia);
- acute lack of oxygen;
with metastases to the bone marrow.
remember that in the case of increased red blood cells, the proportion of reticulocytes can be 50% due to an overestimation of the number of reticulocytes (as noted earlier, reticulocytes are calculated as a % of red blood cells). In cases of severity of anemia, the “reticular index” is calculated by (% reticulocytes x / 45 x where 45 is the hematocrit, 1.85 is the number of reticulocytes required for blood flow. If < 2 indicates a hypoproliferative component of anemia, > 2-3, then increased production of red blood cells [1].
The following drugs can lead to a value of the % content of reticulocytes: antimalarials, antipyretics furazolidone for small children), levodopa.
Remember: reticulocytosis, without erythronormoblastic bone marrow, when areas are irritated by its metastases or foci.
A possible error in reticulocyte counting may be a false overestimation of the presence of:
- inclusions in erythrocytes (Jolly bodies, malarial
- leukocytosis;
- abnormal hemoglobin;
- hyperthrombocytosis;
- giant platelets.
Reticulocytopenia: decreased or absent reticulocytes (reticulocytopenia)
A decrease in the number of reticulocytes (reticulocytopenia) is observed:
- aregenerative aplastic and anemia;
- with anemia, deficiency of vitamin B12, acid (microcytic-hypochromic megaloblastic anemia);
- thalassemia;
- with sideroblastic anemia;
- with bone metastases;
- for illness and therapy;
- with cytostatics;
- autoimmune diseases of the hematopoietic system;
- with kidneys;
- with alcoholism;
- with Addison-Biermer anemia;
Attached files:
-issledovanie_retikulocitov
Principle of the method
- Counting the number of reticulocytes in a smear after staining them with special dyes.
- Counting the number of reticulocytes using fluorescence microscopy.
- Automatic reticulocyte count using a hematology analyzer.
In most domestic laboratories, reticulocytes are counted in a smear after staining them with special dyes. This method is simple, economical, does not require special expensive equipment, and therefore can be used in any clinical diagnostic laboratory.
https://www.youtube.com/watch?v=ytdevru
The second and third methods of counting reticulocytes are also quite simple, but much more expensive from an economic point of view - they require expensive equipment (a fluorescent microscope and a hematology analyzer that counts reticulocytes) and reagents, and therefore can only be used in some laboratories.
Detection of the granular-reticular substance of reticulocytes during supravital staining with alkaline paints with their further counting in a blood smear.
1. Counting the number of reticulocytes in a smear after staining them with special dyes.
This method is in practice the most used method due to the fact that it is simple, fairly cheap and does not require special expensive equipment, and accordingly can be used in any clinical diagnostic laboratory.
The principle of the method is based on identifying the granular-reticular substance of reticulocytes during supravital staining with alkaline paints (saturated solution of brilliant cresyl blue in absolute alcohol, Azur I solution, Azur II solution) with further counting them in a blood smear. Reticulocyte staining is carried out either on glass or in a test tube.
Counting is carried out using a microscope: smears prepared using one of the above methods are microscopically examined with an immersion lens; in the smear, reticulocytes and erythrocytes are colored yellowish-greenish, the granular-filamentous substance in reticulocytes is blue (when stained with azure II and brilliant cresyl blue) or bluish-violet (when stained with azure I).
2. Counting the number of reticulocytes using fluorescence microscopy.
This method is simple and requires little time, it is more accurate than the conventional method, since luminescent microscopy reveals the smallest grains of a mesh-filamentous substance, but it is only possible with a luminescence microscope and special dyes, and therefore is available only to a few laboratories.
https://www.youtube.com/watch?v=ytpolicyandsafetyru
The principle of counting the number of reticulocytes using luminescence microscopy is based on the ability of the reticulocyte substance to fluoresce after treating the blood with acridine orange. Blood is mixed with acridine orange in a test tube or mixers in a ratio of 1 part blood and 10 parts paint.
Microscopy using a ZhS-17 light filter. In the preparation, red blood cells have dark green outlines and do not fluoresce, and in reticulocytes, the granular-mesh substance glows bright red, making reticulocytes easy to count. In blood stabilized with heparin or sodium citrate, reticulocyte fluorescence is not observed.
3. Automatic counting of the number of reticulocytes using a hematology analyzer.
In modern hematology analyzers, the technology for counting blood cells is based on the conductometric method proposed by H. Wallace and Joseph R. Culter in 1947. The principle of the method is to count the number and determine the nature of impulses that occur when a cell passes through a small-diameter hole (aperture), on both sides of which there are two electrodes isolated from each other.
Each passage of a cell through the aperture is accompanied by the appearance of an electrical impulse, which is recorded by an electronic sensor. The division of cells into categories (erythrocytes, leukocytes, platelets, sediment) is carried out by the device based on an analysis of the amplitude of the received pulses. To determine the cell concentration, it is enough to pass a certain volume of sample through the channel and count the number of pulses that are generated.
1. Reticulocytes with a low RNA content, the most mature (LFR%, low fluorescence reticulocyte fractions, fraction of reticulocytes with low fluorescence).
2. Reticulocytes with average RNA content (MFR%, medium fluorescence reticulocyte fractions, fraction of reticulocytes with average fluorescence).
3. Reticulocytes with high RNA content (HFR%, high fluorescence reticulocyte fractions, fraction of reticulocytes with high fluorescence).
4. Immature reticulocyte fraction (IFR%, Immature Reticulocyte Fraction).
https://www.youtube.com/watch?v=ytcreatorsru
The differentiation of reticulocytes, based on the degree of maturity and, accordingly, the content of nucleic acids, is a reflection of the hematopoietic activity of the bone marrow.
Methodology (Sysmex-XT-2000i analyzer). In a flow cell, cells cross a semiconductor laser beam, causing high- and low-angle scattering of the beam and excitation of a fluorescent dye. This makes it possible to determine the different stages of reticulocyte maturity based on the RNA content in the cells and the intensity of their luminescence.
Currently, a unified method is used to count the number of reticulocytes after staining them
- brilliant cresyl blue,
- Azure I or
- Azure II directly on glass or in a test tube.
Principle of the method
Detection of the granular-reticular substance of erythrocytes when stained with alkaline paints with their further counting in a blood smear.
a) a saturated solution of brilliant cresyl blue in absolute alcohol (to prepare absolute alcohol, you need to soak 96% ethanol in several changes of calcined copper sulfate powder): 1.2 g of paint per 100 ml of alcohol;
b) azur I solution: azur I - 1 g, ammonium oxalate - 0.4 g, sodium chloride - 0.8 g, ethyl alcohol 96% - 10 ml, distilled water - 90 ml. The paint solution in a closed bottle is placed in a thermostat at 37 °C for 2–3 days and shaken vigorously periodically. Then cool to room temperature and filter through a paper filter. The solution is stored in a dark glass container. If sediment appears, the paint should be filtered again;
c) Azur II solution: Azur II - 1 g, sodium citrate - 5 g, sodium chloride - 0.4 g, distilled water - 45 ml. The solution is left in a thermostat at 37 °C for 2 days, stirring occasionally. To speed up dissolution, the paint can be heated over low heat for 15–20 minutes, without bringing it to a boil. Cool to room temperature and filter. Store in dark glass containers.
Coloring
- a well-washed and degreased glass slide is heated over a burner flame. Using a glass rod, apply a drop of one of the dyes to the glass and prepare a smear of paint using ground glass. Mark the side of the glass on which the paint stroke is applied with a steklograf. In this form, glass can be prepared for future use and stored in a dry, dark place;
- A drop of blood is applied to the glass prepared in this way, a thin smear is made, and the glass is immediately placed in a damp chamber. To do this, use a Petri dish with a lid, into which slightly moistened rolls of gauze or cotton are placed around the edges;
- The smears are kept in a humid chamber for 3–5 minutes and then dried in air. The granular-mesh substance of reticulocytes is painted violet-blue, clearly standing out against the greenish-bluish background of erythrocytes.
- method 1: before use, prepare a working solution of brilliant cresyl blue in a test tube based on a drop of 1% potassium oxalate solution 4 drops of paint solution 1. Add 40 μl of blood to the paint (two pipettes to the 0.02 mark). The mixture is thoroughly but gently mixed and left for 30 minutes. Stir and prepare thin smears;
- method 2: 0.05 ml of dye solution 3 and 0.2 ml of blood are placed in a test tube. The mixture is thoroughly mixed and left for 20–30 minutes. Stir and prepare thin smears;
- method 3: 0.3–0.5 ml of dye solution 2 and 5–6 drops of blood are placed into a test tube using a pipette from a Panchenkov apparatus. The test tube is closed with a rubber stopper, the mixture is thoroughly but carefully mixed and left for 1–1½ hours (reticulocytes are better stained with an exposure of 1½–3 hours). Mix and prepare thin smears.
- Smears prepared using one of the above methods are examined microscopically with an immersion lens;
- it is necessary to count at least 1000 red blood cells and note among them the number of red blood cells containing a granular-filamentous substance. For uniform thin smears in which the red blood cells are located in one row, select a field of view in which there are, for example, 50 red blood cells, and then calculate 20 such fields of view;
- In practice, for greater accuracy, they use a special eyepiece, in which the field of view can be reduced to the required size. If you don’t have a ready-made eyepiece, you can easily prepare it by unscrewing the ×7 eyepiece, putting a piece of paper with a small square cut out into it and screwing it on. The number of counted reticulocytes is expressed per 1000 or per 100 red blood cells.
Apply 2 drops of a 1% solution of brilliant cresyl blue paint and 1 drop of blood onto a glass slide with a well. Stir carefully with a glass rod and the mixture is placed in a humid chamber (Petri dish, into which lightly moistened rolls of gauze or cotton wool are placed around the edges) for 30 minutes at room temperature. Then smears are made and dried.
Coloring
Literature:
- Directory "Laboratory research methods in the clinic" edited by Menshikov V.V. - Moscow, "Medicine", 1987
- Lyubina A. Ya., Ilyicheva L. P. and co-authors - “Clinical laboratory studies” - Moscow, “Medicine”, 1984.
Reticulocytes are an indicator of the number of cells, the precursors of red blood cells, formed in the bone marrow. Reticulocytes - young red blood cells reflect the regenerative ability of the bone marrow. Reticulocyte counting is used to assess the intensity of hematopoiesis. The study is carried out when: a decrease in the number of red blood cells, suspected hemolysis, assessment of erythropoiesis, in the treatment of anemia with iron supplements and vitamin B 12, erythropoietin.
Reticulocytes are anucleated, immature red blood cells, the maturation of which occurs within 24-48 hours after release into the peripheral blood from the bone marrow. The number of reticulocytes formed reflects the erythropoietic activity of the bone marrow. In other words, the reticulocyte content reflects the rate of red blood cell formation and serves as an indicator of the bone marrow response to anemia.
In addition to the generally accepted parameters of reticulocytes - absolute and relative (%) content of reticulocytes, the emergence of new hematological analyzers (staining with special dyes for nucleic acids) made it possible to obtain additional reticulocyte parameters:
Reticulocytes with low RNA content, the most mature (LFR%, fraction of reticulocytes with low fluorescence); reticulocytes with average RNA content (MFR%, fraction of reticulocytes with average fluorescence); reticulocytes with high RNA content (HFR%, fraction of reticulocytes with high fluorescence); immature reticulocyte fraction (IRF%, Immature Reticulocyte Fraction).
The differentiation of reticulocytes, based on the degree of maturity and, accordingly, the content of nucleic acids, is a reflection of the hematopoietic activity of the bone marrow.
Reagents
You can use one of the following dyes:
- A saturated solution of brilliant cresyl blue in absolute alcohol (to prepare absolute alcohol, you need to soak 96% ethanol in several changes of calcined copper sulfate powder). For 100 ml of absolute alcohol take 1.2 g of paint.
- Solution of azure I: azure I – 1 g, ammonium oxalate – 0.4 g, sodium chloride – 0.8 g, ethyl alcohol 96% – 10 ml, distilled water – 90 ml. The paint solution in a closed bottle is placed in a thermostat at 37° C for 2–3 days and shaken vigorously periodically. Then cool to room temperature and filter through a paper filter. The solution is stored in a dark glass container. If sediment appears, the paint should be filtered again.
- Azur II solution: Azur II – 1 g, sodium citrate – 5 g, sodium chloride – 0.4 g, distilled water – 45 ml. The solution is left in a thermostat at 37° C for 2 days, stirring occasionally. To speed up dissolution, the paint can be heated over low heat for 15 - 20 minutes, without bringing it to a boil. Cool to room temperature and filter. Store in dark glass containers.
Clinical significance of determining the number of reticulocytes
Reticulocyte staining is carried out either on glass or in a test tube.
When staining reticulocytes on glass, a well-washed and degreased glass slide is heated over a burner flame. Using a glass rod, apply a drop of one of the dyes listed above to the glass and prepare a smear of paint using ground glass. A glassograph is used to mark the side of the glass on which a smear of paint is applied.
In this form, glass can be prepared for future use and stored in a dry, dark place. Before analysis, prepare a wet chamber. Typically, a Petri dish with rolls of moistened cotton wool or filter paper placed around the edges is used for this. Apply a drop of blood to a smear of paint, prepare a thin smear from it and immediately place it in a humid chamber for 3 to 10 minutes. Then the smears are air dried.
The staining of reticulocytes in a test tube differs when different dyes are used.
Among the methods for determining hemoglobin, the most widely used are methods based on colorimetry, i.e., determination of color intensity.
The simplest of them is the determination of hemoglobin using visual colorimetry in a Sali hemometer, which is a wooden stand with a central graduated test tube, on the sides of which there are sealed glass tubes with a color standard (hydrochloric acid hematin in glycerin).
A 0.1% solution of hydrochloric acid is first poured into the central test tube to the mark corresponding to 2 or 3 g%, then carefully add (exactly!) 0.02 ml of blood taken from a finger with a special pipette attached to the hemometer. The pipette is washed with the surface layer of acid and, after mixing the blood and acid with a glass rod, it is left for 5 minutes to form hydrochloric acid hematin.
Then, by adding distilled water and constantly stirring with a stick, the color of the liquid in the central test tube completely matches the standards. The hemoglobin concentration corresponds to the level of the solution along the lower meniscus. Hemoglobin concentration can be expressed either in g% hemoglobin or in arbitrary units. 16.67 g of hemoglobin is taken as 100 units.
The concentration of hemoglobin in the blood in women ranges from 11.7 to 15.8 g/o or from 117 to 158 g/l, in men - from 13.3 to 18 g% or from 133 to 180 g/l.
The erythrocyte sedimentation rate is determined in blood mixed in a ratio of 4:1 with sodium citrate.
The reaction is carried out in the Panchenkov apparatus. The Panchenkov capillary is washed with sodium citrate, then the citrate is taken up to the 50 mark, where the letter P (reagent) is written, and it is blown into a Vidalev test tube. Using the same capillary, blood is taken twice up to the K mark (blood) and mixed with citrate. The same capillary is filled with blood mixed with citrate to division 0 and placed vertically in a tripod for an hour.
Normally, ESR in men is 10 mm/h, in women - 14 mm/h.
https://www.youtube.com/watch?v=ytpressru
The erythrocyte sedimentation rate increases in inflammatory, acute and chronic diseases, in malignant tumors and other diseases.